Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Association Between Genetically Proxied Inhibition of HMG-CoA Reductase and Epithelial Ovarian Cancer
JAMA ( IF 63.1 ) Pub Date : 2020-02-18 , DOI: 10.1001/jama.2020.0150 James Yarmolinsky 1, 2 , Caroline J Bull 1, 2, 3 , Emma E Vincent 1, 2, 3 , Jamie Robinson 1, 2 , Axel Walther 4 , George Davey Smith 1, 2 , Sarah J Lewis 1, 2 , Caroline L Relton 1, 2 , Richard M Martin 1, 2, 5
JAMA ( IF 63.1 ) Pub Date : 2020-02-18 , DOI: 10.1001/jama.2020.0150 James Yarmolinsky 1, 2 , Caroline J Bull 1, 2, 3 , Emma E Vincent 1, 2, 3 , Jamie Robinson 1, 2 , Axel Walther 4 , George Davey Smith 1, 2 , Sarah J Lewis 1, 2 , Caroline L Relton 1, 2 , Richard M Martin 1, 2, 5
Affiliation
|
Importance
Preclinical and epidemiological studies indicate a potential chemopreventive role of statins in epithelial ovarian cancer risk. Objective
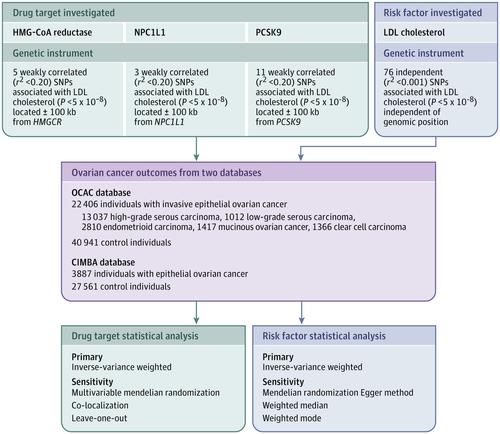
To evaluate the association of genetically proxied inhibition of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (ie, genetic variants related to lower function of HMG-CoA reductase, target of statins) with epithelial ovarian cancer among the general population and in BRCA1/2 mutation carriers. Design, Setting, and Participants
Single-nucleotide polymorphisms (SNPs) in HMGCR, NPC1L1, and PCSK9 associated with low-density lipoprotein (LDL) cholesterol in a genome-wide association study (GWAS) meta-analysis (N ≤196 475) were used to proxy therapeutic inhibition of HMG-CoA reductase, Niemann-Pick C1-Like 1 (NPC1L1) and proprotein convertase subtilisin/kexin type 9 (PCSK9), respectively. Summary statistics were obtained for these SNPs from a GWAS meta-analysis of case-control analyses of invasive epithelial ovarian cancer in the Ovarian Cancer Association Consortium (OCAC; N = 63 347) and from a GWAS meta-analysis of retrospective cohort analyses of epithelial ovarian cancer among BRCA1/2 mutation carriers in the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA; N = 31 448). Across the 2 consortia, participants were enrolled between 1973 and 2014 and followed up through 2015. OCAC participants came from 14 countries and CIMBA participants came from 25 countries. SNPs were combined into multi-allelic models and mendelian randomization estimates representing lifelong inhibition of targets were generated using inverse-variance weighted random-effects models. Exposures
Primary exposure was genetically proxied inhibition of HMG-CoA reductase and secondary exposures were genetically proxied inhibition of NPC1L1 and PCSK9 and genetically proxied circulating LDL cholesterol levels. Main Outcomes and Measures
Overall and histotype-specific invasive epithelial ovarian cancer (general population) and epithelial ovarian cancer (BRCA1/2 mutation carriers), measured as ovarian cancer odds (general population) and hazard ratio (BRCA1/2 mutation carriers). Results
The OCAC sample included 22 406 women with invasive epithelial ovarian cancer and 40 941 control individuals and the CIMBA sample included 3887 women with epithelial ovarian cancer and 27 561 control individuals. Median ages for the cohorts ranged from 41.5 to 59.0 years and all participants were of European ancestry. In the primary analysis, genetically proxied HMG-CoA reductase inhibition equivalent to a 1-mmol/L (38.7-mg/dL) reduction in LDL cholesterol was associated with lower odds of epithelial ovarian cancer (odds ratio [OR], 0.60 [95% CI, 0.43-0.83]; P = .002). In BRCA1/2 mutation carriers, genetically proxied HMG-CoA reductase inhibition was associated with lower ovarian cancer risk (hazard ratio, 0.69 [95% CI, 0.51-0.93]; P = .01). In secondary analyses, there were no significant associations of genetically proxied inhibition of NPC1L1 (OR, 0.97 [95% CI, 0.53-1.75]; P = .91), PCSK9 (OR, 0.98 [95% CI, 0.85-1.13]; P = .80), or circulating LDL cholesterol (OR, 0.98 [95% CI, 0.91-1.05]; P = .55) with epithelial ovarian cancer. Conclusions and Relevance
Genetically proxied inhibition of HMG-CoA reductase was significantly associated with lower odds of epithelial ovarian cancer. However, these findings do not indicate risk reduction from medications that inhibit HMG-CoA reductase; further research is needed to understand whether there is a similar association with such medications.
中文翻译:

HMG-CoA 还原酶的基因代理抑制与上皮性卵巢癌之间的关联
重要性 临床前和流行病学研究表明他汀类药物对上皮性卵巢癌风险具有潜在的化学预防作用。目的 评估 3-羟基-3-甲基戊二酰辅酶 A (HMG-CoA) 还原酶的遗传代理抑制(即与他汀类药物靶点 HMG-CoA 还原酶功能较低相关的遗传变异)与上皮性卵巢癌的关系。一般人群和 BRCA1/2 突变携带者。设计、设置和参与者 在全基因组关联研究 (GWAS) 荟萃分析 (N ≤196 475) 中,HMGCR、NPC1L1 和 PCSK9 中与低密度脂蛋白 (LDL) 胆固醇相关的单核苷酸多态性 (SNP) 被分别用于代理 HMG-CoA 还原酶、Niemann-Pick C1-Like 1 (NPC1L1) 和前蛋白转化酶枯草杆菌蛋白酶/kexin 9 型 (PCSK9) 的治疗性抑制。这些 SNP 的汇总统计数据来自卵巢癌协会联盟 (OCAC;N = 63 347) 侵袭性上皮性卵巢癌病例对照分析的 GWAS 荟萃分析以及上皮性上皮性卵巢癌回顾性队列分析的 GWAS 荟萃分析。 BRCA1/2 修饰研究者联盟 (CIMBA;N = 31 448) 中 BRCA1/2 突变携带者的卵巢癌。在这两个联盟中,参与者在 1973 年至 2014 年期间注册,并一直持续到 2015 年。OCAC 参与者来自 14 个国家,CIMBA 参与者来自 25 个国家。 SNP 被组合成多等位基因模型,并使用逆方差加权随机效应模型生成代表靶标终身抑制的孟德尔随机化估计。 暴露 主要暴露是 HMG-CoA 还原酶的遗传代理抑制,二次暴露是 NPC1L1 和 PCSK9 的遗传代理抑制以及循环 LDL 胆固醇水平的遗传代理抑制。主要结果和措施 总体和组织型特异性浸润性上皮性卵巢癌(一般人群)和上皮性卵巢癌(BRCA1/2 突变携带者),以卵巢癌几率(一般人群)和风险比(BRCA1/2 突变携带者)来衡量。结果 OCAC 样本包括 22 406 名患有浸润性上皮性卵巢癌的女性和 40 941 名对照个体,CIMBA 样本包括 3887 名患有上皮性卵巢癌的女性和 27 561 名对照个体。该队列的中位年龄为 41.5 岁至 59.0 岁,所有参与者均为欧洲血统。在初步分析中,遗传代理的 HMG-CoA 还原酶抑制相当于 LDL 胆固醇降低 1 mmol/L (38.7 mg/dL),与较低的上皮性卵巢癌发病率相关(比值比 [OR],0.60 [95] %CI,0.43-0.83];P = .002)。在 BRCA1/2 突变携带者中,基因代理的 HMG-CoA 还原酶抑制与较低的卵巢癌风险相关(风险比,0.69 [95% CI,0.51-0.93];P = 0.01)。在二次分析中,NPC1L1(OR,0.97 [95% CI,0.53-1.75];P = 0.91)、PCSK9(OR,0.98 [95% CI,0.85-1.13];P = 0.91)之间没有显着关联。 P = .80) 或循环 LDL 胆固醇 (OR, 0.98 [95% CI, 0.91-1.05]; P = .55) 与上皮性卵巢癌的关系。结论和相关性 HMG-CoA 还原酶的遗传代理抑制与上皮性卵巢癌的较低几率显着相关。 然而,这些发现并不表明抑制 HMG-CoA 还原酶的药物可以降低风险;需要进一步的研究来了解是否与此类药物存在类似的关联。
更新日期:2020-02-18
中文翻译:

HMG-CoA 还原酶的基因代理抑制与上皮性卵巢癌之间的关联
重要性 临床前和流行病学研究表明他汀类药物对上皮性卵巢癌风险具有潜在的化学预防作用。目的 评估 3-羟基-3-甲基戊二酰辅酶 A (HMG-CoA) 还原酶的遗传代理抑制(即与他汀类药物靶点 HMG-CoA 还原酶功能较低相关的遗传变异)与上皮性卵巢癌的关系。一般人群和 BRCA1/2 突变携带者。设计、设置和参与者 在全基因组关联研究 (GWAS) 荟萃分析 (N ≤196 475) 中,HMGCR、NPC1L1 和 PCSK9 中与低密度脂蛋白 (LDL) 胆固醇相关的单核苷酸多态性 (SNP) 被分别用于代理 HMG-CoA 还原酶、Niemann-Pick C1-Like 1 (NPC1L1) 和前蛋白转化酶枯草杆菌蛋白酶/kexin 9 型 (PCSK9) 的治疗性抑制。这些 SNP 的汇总统计数据来自卵巢癌协会联盟 (OCAC;N = 63 347) 侵袭性上皮性卵巢癌病例对照分析的 GWAS 荟萃分析以及上皮性上皮性卵巢癌回顾性队列分析的 GWAS 荟萃分析。 BRCA1/2 修饰研究者联盟 (CIMBA;N = 31 448) 中 BRCA1/2 突变携带者的卵巢癌。在这两个联盟中,参与者在 1973 年至 2014 年期间注册,并一直持续到 2015 年。OCAC 参与者来自 14 个国家,CIMBA 参与者来自 25 个国家。 SNP 被组合成多等位基因模型,并使用逆方差加权随机效应模型生成代表靶标终身抑制的孟德尔随机化估计。 暴露 主要暴露是 HMG-CoA 还原酶的遗传代理抑制,二次暴露是 NPC1L1 和 PCSK9 的遗传代理抑制以及循环 LDL 胆固醇水平的遗传代理抑制。主要结果和措施 总体和组织型特异性浸润性上皮性卵巢癌(一般人群)和上皮性卵巢癌(BRCA1/2 突变携带者),以卵巢癌几率(一般人群)和风险比(BRCA1/2 突变携带者)来衡量。结果 OCAC 样本包括 22 406 名患有浸润性上皮性卵巢癌的女性和 40 941 名对照个体,CIMBA 样本包括 3887 名患有上皮性卵巢癌的女性和 27 561 名对照个体。该队列的中位年龄为 41.5 岁至 59.0 岁,所有参与者均为欧洲血统。在初步分析中,遗传代理的 HMG-CoA 还原酶抑制相当于 LDL 胆固醇降低 1 mmol/L (38.7 mg/dL),与较低的上皮性卵巢癌发病率相关(比值比 [OR],0.60 [95] %CI,0.43-0.83];P = .002)。在 BRCA1/2 突变携带者中,基因代理的 HMG-CoA 还原酶抑制与较低的卵巢癌风险相关(风险比,0.69 [95% CI,0.51-0.93];P = 0.01)。在二次分析中,NPC1L1(OR,0.97 [95% CI,0.53-1.75];P = 0.91)、PCSK9(OR,0.98 [95% CI,0.85-1.13];P = 0.91)之间没有显着关联。 P = .80) 或循环 LDL 胆固醇 (OR, 0.98 [95% CI, 0.91-1.05]; P = .55) 与上皮性卵巢癌的关系。结论和相关性 HMG-CoA 还原酶的遗传代理抑制与上皮性卵巢癌的较低几率显着相关。 然而,这些发现并不表明抑制 HMG-CoA 还原酶的药物可以降低风险;需要进一步的研究来了解是否与此类药物存在类似的关联。











































 京公网安备 11010802027423号
京公网安备 11010802027423号