Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sustainable Low-Concentration Arsenite [As(III)] Removal in Single and Multicomponent Systems Using Hybrid Iron Oxide-Biochar Nanocomposite Adsorbents-A Mechanistic Study.
ACS Omega ( IF 3.7 ) Pub Date : 2020-02-06 , DOI: 10.1021/acsomega.9b02842 Prachi Singh 1 , Ankur Sarswat 1 , Charles U Pittman 2 , Todd Mlsna 2 , Dinesh Mohan 1
ACS Omega ( IF 3.7 ) Pub Date : 2020-02-06 , DOI: 10.1021/acsomega.9b02842 Prachi Singh 1 , Ankur Sarswat 1 , Charles U Pittman 2 , Todd Mlsna 2 , Dinesh Mohan 1
Affiliation
|
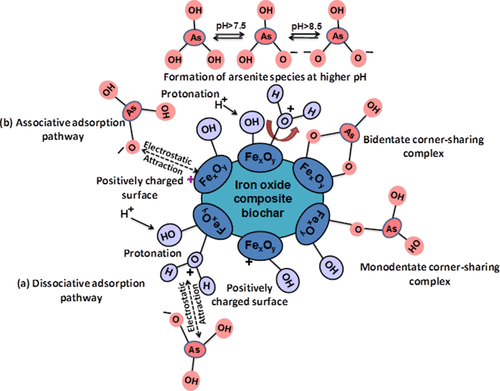
Rice and wheat husks were converted to biochars by slow pyrolysis (1 h) at 600 °C. Iron oxide rice husk hybrid biochar (RHIOB) and wheat husk hybrid biochar (WHIOB) were synthesized by copyrolysis of FeCl3-impregnated rice or wheat husks at 600 °C. These hybrid sorbents were characterized using X-ray photoelectron spectroscopy, X-ray diffraction, scanning electron microscopy (SEM), SEM-energy-dispersive X-ray spectroscopy, Fourier transform infrared spectroscopy, transmission electron microscopy, physical parameter measurement system, and Brunauer-Emmett-Teller (BET) surface area techniques. Fe3O4 was the predominant iron oxide present with some Fe2O3. RHIOB and WHIOB rapidly chemisorbed As(III) from water (∼24% removal in first half an hour reaching up to ∼100% removal in 24 h) at surface Fe-OH functions forming monodentate ≡Fe-OAs(OH)2 and bidentate (≡Fe-O)2AsOH complexes. Optimum removal occurred in the pH 7.5-8.5 range for both RHIOB and WHIOB, but excellent removal occurred from pH 3 to 10. Batch kinetic studies at various initial adsorbate-adsorbent concentrations, temperatures, and contact times gave excellent pseudo-second-order model fits. Equilibrium data were fitted to different sorption isotherm models. Fits to isotherm models (based on R 2 and χ2) on RHIOB and WHIOB followed the order: Redlich-Peterson > Toth > Sips = Koble-Corrigan > Langmuir > Freundlich = Radke-Prausnitz > Temkin and Sips = Koble-Corrigan > Toth > Redlich-Peterson > Langmuir > Temkin > Freundlich = Radke-Prausnitz, respectively. Maximum adsorption capacities, Q RHIOB 0 = 96 μg/g and Q WHIOB 0 = 111 μg/g, were obtained. No As(III) oxidation to As(V) was detected. Arsenic adsorption was endothermic. Particle diffusion was a rate-determining step at low (≤50 μg/L) concentrations, but film diffusion controls the rate at ≥100-200 μg/L. Binding interactions with RHIOB and WHIOB were established, and the mechanism was carefully discussed. RHIOB and WHIOB can successfully be used for As(III) removal in single and multicomponent systems with no significant decrease in adsorption capacity in the presence of interfering ions mainly Cl-, HCO3 -, NO3 -, SO4 2-, PO4 3-, K+, Na+, Ca2+. Simultaneous As(III) desorption and regeneration of RHIOB and WHIOB was successfully achieved. A very nominal decrease in As(III) removal capacity in four consecutive cycles demonstrates the reusability of RHIOB and WHIOB. Furthermore, these sustainable composites had good sorption efficiencies and may be removed magnetically to avoid slow filtration.
中文翻译:

混合氧化铁-生物炭纳米复合吸附剂在单组分和多组分体系中可持续去除低浓度砷[As(III)]的机理研究。
通过在600°C下缓慢热解(1 h),将稻壳和小麦壳转化为生物炭。通过在600°C下对FeCl3浸渍的稻壳或小麦壳进行共水解,合成了氧化铁稻壳杂交生物碳(RHIOB)和小麦壳杂交生物碳(WHIOB)。使用X射线光电子能谱,X射线衍射,扫描电子显微镜(SEM),SEM能量色散X射线光谱,傅里叶变换红外光谱,透射电子显微镜,物理参数测量系统和Brunauer来表征这些杂化吸附剂-Emmett-Teller(BET)表面积技术。Fe3O4是与某些Fe2O3一起存在的主要氧化铁。RHIOB和WHIOB在表面Fe-OH上迅速从水中化学吸附了As(III)(前半小时约24%去除,到24小时内约100%去除),形成单齿≡Fe-OAs(OH)2和二齿(≡Fe-O)2AsOH配合物。RHIOB和WHIOB的最佳去除均在pH 7.5-8.5范围内,但在pH 3到10范围内均达到最佳去除效果。在各种初始吸附物-吸附剂浓度,温度和接触时间下进行的批动力学研究提供了出色的拟二级模型适合。平衡数据适合不同的吸附等温线模型。在RHIOB和WHIOB上拟合等温模型(基于R 2和χ2)的顺序如下:Redlich-Peterson> Toth> Sips = Koble-Corrigan> Langmuir> Freundlich = Radke-Prausnitz> Temkin和Sips = Koble-Corrigan> Toth>雷德利希·彼得森> Langmuir> Temkin> Freundlich = Radke-Prausnitz。获得最大吸附容量,Q RHIOB 0 = 96μg/ g和Q WHIOB 0 = 111μg/ g。没有检测到As(III)氧化为As(V)。砷吸附是吸热的。在低浓度(≤50μg/ L)下,颗粒扩散是一个决定速率的步骤,但薄膜扩散将速率控制在≥100-200μg/ L。建立了与RHIOB和WHIOB的结合相互作用,并仔细讨论了该机制。RHIOB和WHIOB可以成功用于单组分和多组分系统中的As(III)去除,并且在存在主要为Cl-,HCO3-,NO3-,SO4 2-,PO4 3-,K +的干扰离子的情况下,吸附能力不会显着降低。 ,Na +,Ca2 +。成功实现了RHIOB和WHIOB的同时As(III)解吸和再生。在四个连续的循环中,砷(III)去除能力的非常名义上的下降证明了RHIOB和WHIOB的可重用性。此外,这些可持续的复合材料具有良好的吸附效率,可以通过磁力去除,以免缓慢过滤。
更新日期:2020-02-18
中文翻译:

混合氧化铁-生物炭纳米复合吸附剂在单组分和多组分体系中可持续去除低浓度砷[As(III)]的机理研究。
通过在600°C下缓慢热解(1 h),将稻壳和小麦壳转化为生物炭。通过在600°C下对FeCl3浸渍的稻壳或小麦壳进行共水解,合成了氧化铁稻壳杂交生物碳(RHIOB)和小麦壳杂交生物碳(WHIOB)。使用X射线光电子能谱,X射线衍射,扫描电子显微镜(SEM),SEM能量色散X射线光谱,傅里叶变换红外光谱,透射电子显微镜,物理参数测量系统和Brunauer来表征这些杂化吸附剂-Emmett-Teller(BET)表面积技术。Fe3O4是与某些Fe2O3一起存在的主要氧化铁。RHIOB和WHIOB在表面Fe-OH上迅速从水中化学吸附了As(III)(前半小时约24%去除,到24小时内约100%去除),形成单齿≡Fe-OAs(OH)2和二齿(≡Fe-O)2AsOH配合物。RHIOB和WHIOB的最佳去除均在pH 7.5-8.5范围内,但在pH 3到10范围内均达到最佳去除效果。在各种初始吸附物-吸附剂浓度,温度和接触时间下进行的批动力学研究提供了出色的拟二级模型适合。平衡数据适合不同的吸附等温线模型。在RHIOB和WHIOB上拟合等温模型(基于R 2和χ2)的顺序如下:Redlich-Peterson> Toth> Sips = Koble-Corrigan> Langmuir> Freundlich = Radke-Prausnitz> Temkin和Sips = Koble-Corrigan> Toth>雷德利希·彼得森> Langmuir> Temkin> Freundlich = Radke-Prausnitz。获得最大吸附容量,Q RHIOB 0 = 96μg/ g和Q WHIOB 0 = 111μg/ g。没有检测到As(III)氧化为As(V)。砷吸附是吸热的。在低浓度(≤50μg/ L)下,颗粒扩散是一个决定速率的步骤,但薄膜扩散将速率控制在≥100-200μg/ L。建立了与RHIOB和WHIOB的结合相互作用,并仔细讨论了该机制。RHIOB和WHIOB可以成功用于单组分和多组分系统中的As(III)去除,并且在存在主要为Cl-,HCO3-,NO3-,SO4 2-,PO4 3-,K +的干扰离子的情况下,吸附能力不会显着降低。 ,Na +,Ca2 +。成功实现了RHIOB和WHIOB的同时As(III)解吸和再生。在四个连续的循环中,砷(III)去除能力的非常名义上的下降证明了RHIOB和WHIOB的可重用性。此外,这些可持续的复合材料具有良好的吸附效率,可以通过磁力去除,以免缓慢过滤。









































 京公网安备 11010802027423号
京公网安备 11010802027423号