Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transfer Hydrogenation of Ketones Catalyzed by Symmetric Imino-N-heterocyclic Carbene Co(III) Complexes.
ACS Omega ( IF 3.7 ) Pub Date : 2020-02-06 , DOI: 10.1021/acsomega.9b03181 Samaila Abubakar 1 , Muhammad D Bala 1
ACS Omega ( IF 3.7 ) Pub Date : 2020-02-06 , DOI: 10.1021/acsomega.9b03181 Samaila Abubakar 1 , Muhammad D Bala 1
Affiliation
|
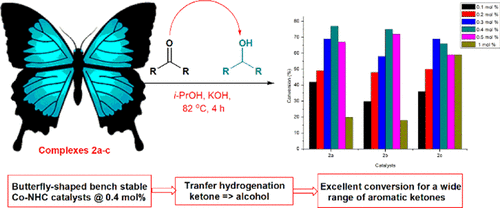
The synthesis of new moisture-sensitive imine-functionalized N-heterocyclic carbene (NHC) precursor salts [1-(2-[(hydroxyl-benzylidene)-amino]-ethyl)-3-R-3H-imidazole-1-ium bromide; R = methyl (1a), ethyl (1b), and benzyl (1c)] is reported. Subsequent deprotonation of 1a-c and coordination of the in situ generated NHC ligands to CoBr2 led to the isolation of air-stable six-coordinate Co(III) complexes 2a-c, respectively. All the salts and complexes were fully characterized. Single-crystal X-ray analysis of 2a and 2c showed octahedral Co centers hexacoordinated to two NHC carbons, two imine nitrogen atoms, and two phenolate oxygens in the form [C^N^O(Co3+)C^N^O]. The complexes were used in the catalytic transfer hydrogenation (CTH) of a range of ketones in 2-propanol as the solvent and hydrogen donor. Based on a low catalyst concentration of 0.4 mol %, significant conversions in the range of 70-99% were recorded at high turnover frequencies up to 1635 h-1. A mechanism to account for the steps involved in the CTH of cyclohexanone by complex 2a is proposed and supported by data from cyclic voltammetry, low-resolution mass spectrometry, UV, and IR spectroscopic techniques.
中文翻译:

对称氨基-N-杂环碳烯Co(III)配合物催化的酮的转移加氢
新型湿敏亚胺官能化的N-杂环卡宾(NHC)前体盐[1-(2-[(羟基-亚苄基)-氨基]-乙基)-3-R-3H-咪唑-1-溴化铵的合成; R =甲基(1a),乙基(1b)和苄基(1c)]。随后1a-c的去质子化和原位生成的NHC配体与CoBr2的配位导致分别分离出空气稳定的六配位Co(III)配合物2a-c。所有的盐和配合物均已充分表征。2a和2c的单晶X射线分析表明,八面体Co中心与两个NHC碳,两个亚胺氮原子和两个酚盐氧六配位,形式为[C ^ N ^ O(Co3 +)C ^ N ^ O]。将该配合物用于2-丙醇中作为溶剂和氢供体的一系列酮的催化转移氢化(CTH)。基于0的低催化剂浓度。在高达1635 h-1的高周转频率下,记录到4 mol%的显着转化率在70-99%范围内。提出了一种机制来解释配合物2a参与环己酮CTH的步骤,并得到循环伏安法,低分辨率质谱法,UV和IR光谱技术的数据的支持。
更新日期:2020-02-18
中文翻译:

对称氨基-N-杂环碳烯Co(III)配合物催化的酮的转移加氢
新型湿敏亚胺官能化的N-杂环卡宾(NHC)前体盐[1-(2-[(羟基-亚苄基)-氨基]-乙基)-3-R-3H-咪唑-1-溴化铵的合成; R =甲基(1a),乙基(1b)和苄基(1c)]。随后1a-c的去质子化和原位生成的NHC配体与CoBr2的配位导致分别分离出空气稳定的六配位Co(III)配合物2a-c。所有的盐和配合物均已充分表征。2a和2c的单晶X射线分析表明,八面体Co中心与两个NHC碳,两个亚胺氮原子和两个酚盐氧六配位,形式为[C ^ N ^ O(Co3 +)C ^ N ^ O]。将该配合物用于2-丙醇中作为溶剂和氢供体的一系列酮的催化转移氢化(CTH)。基于0的低催化剂浓度。在高达1635 h-1的高周转频率下,记录到4 mol%的显着转化率在70-99%范围内。提出了一种机制来解释配合物2a参与环己酮CTH的步骤,并得到循环伏安法,低分辨率质谱法,UV和IR光谱技术的数据的支持。











































 京公网安备 11010802027423号
京公网安备 11010802027423号