当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recycling of CO2 by Hydrogenation of Carbonate Derivatives to Methanol: Tuning Copper-Oxide Promotion Effects in Supported Catalysts.
ChemSusChem ( IF 7.5 ) Pub Date : 2020-03-13 , DOI: 10.1002/cssc.202000166 Jonglack Kim 1 , Norbert Pfänder 2 , Gonzalo Prieto 1
ChemSusChem ( IF 7.5 ) Pub Date : 2020-03-13 , DOI: 10.1002/cssc.202000166 Jonglack Kim 1 , Norbert Pfänder 2 , Gonzalo Prieto 1
Affiliation
|
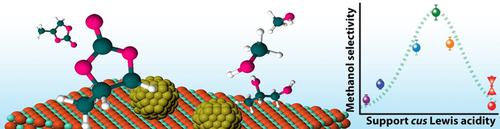
The selective hydrogenation of organic carbonates to methanol is a relevant transformation to realize flexible processes for the recycling of waste CO2 with renewable H2 mediated by condensed carbon dioxide surrogates. Oxide-supported copper nanoparticles are promising solid catalysts for this selective hydrogenation. However, essential for their optimization is to rationalize the prominent impact of the oxide support on performance. Herein, the role of Lewis acid centers, exposed on the oxide support at the periphery of the Cu nanoparticles, was systematically assessed. For the hydrogenation of propylene carbonate, as a model cyclic carbonate, the conversion rate, the apparent activation energy, and the selectivity to methanol correlate with the Lewis acidity of the coordinatively unsaturated cationic sites exposed on the oxide support. Lewis sites of markedly low and high electron-withdrawing character promote unselective decarbonylation and decarboxylation reaction pathways, respectively. Supports exposing Lewis sites of intermediate acidity maximize the selectivity to methanol while inhibiting acid-catalyzed secondary reactions of the propanediol product, and thus enable its recovery in cyclic processes of CO2 hydrogenation mediated by condensed carbonate derivatives. These findings help rationalize metal-support promotion effects that determine the performance of supported metal nanoparticles in this and other selective hydrogenation reactions of significance in the context of sustainable chemistry.
中文翻译:

通过将碳酸酯衍生物氢化为甲醇来回收CO2:调节负载型催化剂中铜氧化物的促进作用。
将有机碳酸盐选择性氢化为甲醇是一项重要的转变,目的是实现灵活的过程,以利用冷凝的二氧化碳替代物介导的可再生H2回收废CO2。氧化物负载的铜纳米颗粒是用于这种选择性氢化的有前途的固体催化剂。然而,优化它们的关键是使氧化物载体对性能的显着影响合理化。在此,系统地评估了暴露在Cu纳米颗粒外围的氧化物载体上的Lewis酸中心的作用。对于作为模型环状碳酸酯的碳酸亚丙酯的氢化,转化率,表观活化能和对甲醇的选择性与暴露于氧化物载体上的配位不饱和阳离子位的路易斯酸度相关。具有明显低吸电子特性的路易斯位点分别促进非选择性脱羰和脱羧反应途径。暴露出具有中等酸性的Lewis位点的载体可最大程度地提高对甲醇的选择性,同时抑制丙二醇产物的酸催化副反应,因此可使其在缩合碳酸盐衍生物介导的CO2加氢循环过程中回收。这些发现有助于合理化金属载体的促进作用,这种作用决定了在可持续化学的背景下,在这种和其他选择性氢化反应中具有重要意义的载体金属纳米颗粒的性能。暴露出具有中等酸性的Lewis位点的载体可最大程度地提高对甲醇的选择性,同时抑制丙二醇产物的酸催化副反应,因此可使其在缩合碳酸盐衍生物介导的CO2加氢循环过程中回收。这些发现有助于合理化金属载体的促进作用,这种作用决定了在可持续化学的背景下,在这种和其他选择性氢化反应中具有重要意义的载体金属纳米颗粒的性能。暴露出具有中等酸性的Lewis位点的载体可最大程度地提高对甲醇的选择性,同时抑制丙二醇产物的酸催化副反应,因此可使其在缩合碳酸盐衍生物介导的CO2加氢循环过程中回收。这些发现有助于合理化金属载体的促进作用,这种作用决定了在可持续化学的背景下,在这种和其他选择性氢化反应中具有重要意义的载体金属纳米颗粒的性能。
更新日期:2020-04-22
中文翻译:

通过将碳酸酯衍生物氢化为甲醇来回收CO2:调节负载型催化剂中铜氧化物的促进作用。
将有机碳酸盐选择性氢化为甲醇是一项重要的转变,目的是实现灵活的过程,以利用冷凝的二氧化碳替代物介导的可再生H2回收废CO2。氧化物负载的铜纳米颗粒是用于这种选择性氢化的有前途的固体催化剂。然而,优化它们的关键是使氧化物载体对性能的显着影响合理化。在此,系统地评估了暴露在Cu纳米颗粒外围的氧化物载体上的Lewis酸中心的作用。对于作为模型环状碳酸酯的碳酸亚丙酯的氢化,转化率,表观活化能和对甲醇的选择性与暴露于氧化物载体上的配位不饱和阳离子位的路易斯酸度相关。具有明显低吸电子特性的路易斯位点分别促进非选择性脱羰和脱羧反应途径。暴露出具有中等酸性的Lewis位点的载体可最大程度地提高对甲醇的选择性,同时抑制丙二醇产物的酸催化副反应,因此可使其在缩合碳酸盐衍生物介导的CO2加氢循环过程中回收。这些发现有助于合理化金属载体的促进作用,这种作用决定了在可持续化学的背景下,在这种和其他选择性氢化反应中具有重要意义的载体金属纳米颗粒的性能。暴露出具有中等酸性的Lewis位点的载体可最大程度地提高对甲醇的选择性,同时抑制丙二醇产物的酸催化副反应,因此可使其在缩合碳酸盐衍生物介导的CO2加氢循环过程中回收。这些发现有助于合理化金属载体的促进作用,这种作用决定了在可持续化学的背景下,在这种和其他选择性氢化反应中具有重要意义的载体金属纳米颗粒的性能。暴露出具有中等酸性的Lewis位点的载体可最大程度地提高对甲醇的选择性,同时抑制丙二醇产物的酸催化副反应,因此可使其在缩合碳酸盐衍生物介导的CO2加氢循环过程中回收。这些发现有助于合理化金属载体的促进作用,这种作用决定了在可持续化学的背景下,在这种和其他选择性氢化反应中具有重要意义的载体金属纳米颗粒的性能。









































 京公网安备 11010802027423号
京公网安备 11010802027423号