当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereoselective Synthesis of Benzoxanthenones.
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-02-16 , DOI: 10.1002/asia.201901727 William C Neuhaus 1 , Marisa C Kozlowski 1
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-02-16 , DOI: 10.1002/asia.201901727 William C Neuhaus 1 , Marisa C Kozlowski 1
Affiliation
|
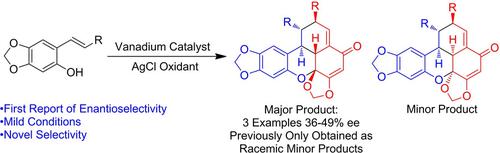
An oxidative catalytic vanadium(V) system was developed to access to the naturally nonabundant diastereomers of carpanone from the corresponding alkenyl phenol monomer in one pot by tandem oxidation, oxidative coupling, and 4+2 cyclization. This system was applied to synthesize two other analogues of carpanone. Mild oxidizing silver salts were used as the terminal oxidant to minimize background oxidation which produces the natural diastereomer of carpanone. Further, the first examples of enantioselective oxidative benzoxanthenone formation are reported. Solvent polarity has a strong effect on enantioselectivity, consistent with a mechanism involving binding of vanadium Schiff base catalysts to the alcoholic moiety of the alkenyl phenols during the cyclization step.
中文翻译:

苯并氧杂蒽酮的非对映选择性合成。
通过串联氧化,氧化偶联和4 + 2环化反应,开发了一种氧化催化钒(V)系统,可从一锅中相应的烯基苯酚单体中获得呋喃酮的天然非丰富非对映异构体。该系统被用于合成另外两个甲酮的类似物。使用轻度氧化的银盐作为末端氧化剂,以最大程度地减少背景氧化,而背景氧化会生成天然的甲酮非对映异构体。此外,报道了对映选择性氧化苯并氧杂蒽酮形成的第一个实例。溶剂极性对对映选择性有很强的影响,这与在环化步骤中涉及钒席夫碱催化剂与烯基酚的醇部分结合的机理一致。
更新日期:2020-03-12
中文翻译:

苯并氧杂蒽酮的非对映选择性合成。
通过串联氧化,氧化偶联和4 + 2环化反应,开发了一种氧化催化钒(V)系统,可从一锅中相应的烯基苯酚单体中获得呋喃酮的天然非丰富非对映异构体。该系统被用于合成另外两个甲酮的类似物。使用轻度氧化的银盐作为末端氧化剂,以最大程度地减少背景氧化,而背景氧化会生成天然的甲酮非对映异构体。此外,报道了对映选择性氧化苯并氧杂蒽酮形成的第一个实例。溶剂极性对对映选择性有很强的影响,这与在环化步骤中涉及钒席夫碱催化剂与烯基酚的醇部分结合的机理一致。









































 京公网安备 11010802027423号
京公网安备 11010802027423号