当前位置:
X-MOL 学术
›
Cell Discov.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and functional insight into the effect of AFF4 dimerization on activation of HIV-1 proviral transcription.
Cell Discovery ( IF 13.0 ) Pub Date : 2020-02-18 , DOI: 10.1038/s41421-020-0142-6 Dan Tang 1 , Chunjing Chen 2 , Ga Liao 3 , Jiaming Liu 1 , Banghua Liao 1 , QingQing Huang 2 , Qianqian Chen 1 , Jiahui Zhao 1 , Hui Jiang 1 , Jinsong Duan 4 , Jin Huang 1 , Kunjie Wang 1 , Jiawei Wang 4 , Cuiyan Zhou 5 , Wendan Chu 5 , Wenqi Li 5 , Bo Sun 6 , Zhonghan Li 1 , Lunzhi Dai 1 , Xianghui Fu 1 , Wei Cheng 1 , Yuhua Xue 2 , Shiqian Qi 1
Cell Discovery ( IF 13.0 ) Pub Date : 2020-02-18 , DOI: 10.1038/s41421-020-0142-6 Dan Tang 1 , Chunjing Chen 2 , Ga Liao 3 , Jiaming Liu 1 , Banghua Liao 1 , QingQing Huang 2 , Qianqian Chen 1 , Jiahui Zhao 1 , Hui Jiang 1 , Jinsong Duan 4 , Jin Huang 1 , Kunjie Wang 1 , Jiawei Wang 4 , Cuiyan Zhou 5 , Wendan Chu 5 , Wenqi Li 5 , Bo Sun 6 , Zhonghan Li 1 , Lunzhi Dai 1 , Xianghui Fu 1 , Wei Cheng 1 , Yuhua Xue 2 , Shiqian Qi 1
Affiliation
|
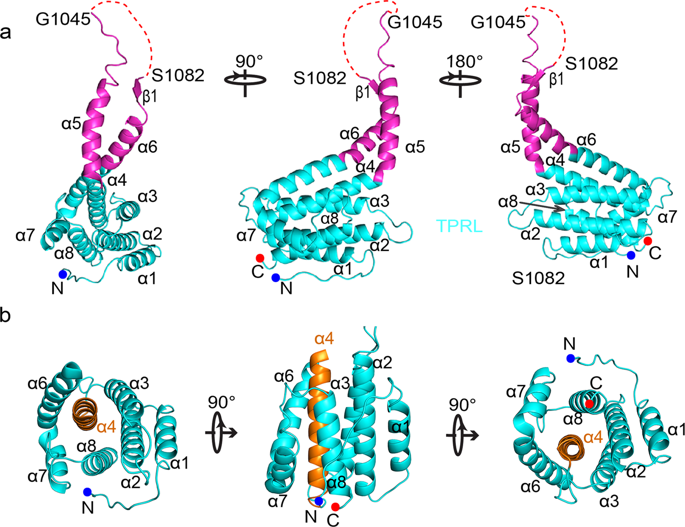
Super elongation complex (SEC) is a positive regulator of RNA polymerase II, which is required for HIV-1 proviral transcription. AFF1/4 is the scaffold protein that recruits other components of SEC and forms dimer depending on its THD domain (TPRL with Handle Region Dimerization Domain). Here we report the crystal structure of the human AFF4-THD at the resolution of 2.4 Å. The α4, α5, and α6 of one AFF4-THD mediate the formation of a dimer and pack tightly against the equivalent part of the second molecule in the dimer of AFF-THD. Mutagenesis analysis revealed that single mutations of either Phe1014 or Tyr1096 of AFF4 to alanine impair the formation of the AFF4 dimer. In addition, transactivation assay also indicated that Phe1014 and Tyr1096 of AFF4 are critical to the transactivation activity of AFF4. Interestingly, the corresponding residues Phe1063 and Tyr1145 in AFF1 have an effect on the transactivation of HIV-1 provirus. However, such mutations of AFF1/4 have no effect on the interaction of AFF1/4 with other subunits of the SEC. Together, our data demonstrated that the dimerization of AFF1/4 is essential to transactivation of HIV-1 provirus.
中文翻译:

深入了解 AFF4 二聚化对 HIV-1 前病毒转录激活的影响。
超级延伸复合物 (SEC) 是 RNA 聚合酶 II 的正调节因子,而 RNA 聚合酶 II 是 HIV-1 原病毒转录所必需的。AFF1/4 是一种支架蛋白,它招募 SEC 的其他组件并根据其 THD 结构域(具有处理区域二聚化结构域的 TPRL)形成二聚体。在这里,我们以 2.4 Å 的分辨率报告了人类 AFF4-THD 的晶体结构。一个 AFF4-THD 的 α4、α5 和 α6 介导二聚体的形成,并与 AFF-THD 二聚体中第二个分子的等效部分紧密堆积。诱变分析表明,AFF4 的 Phe1014 或 Tyr1096 单一突变为丙氨酸会损害 AFF4 二聚体的形成。此外,反式激活实验还表明AFF4的Phe1014和Tyr1096对于AFF4的反式激活活性至关重要。有趣的是,AFF1中相应的残基Phe1063和Tyr1145对HIV-1原病毒的反式激活有影响。然而,AFF1/4 的此类突变对 AFF1/4 与 SEC 其他亚基的相互作用没有影响。总之,我们的数据表明 AFF1/4 的二聚化对于 HIV-1 原病毒的反式激活至关重要。
更新日期:2020-02-18
中文翻译:

深入了解 AFF4 二聚化对 HIV-1 前病毒转录激活的影响。
超级延伸复合物 (SEC) 是 RNA 聚合酶 II 的正调节因子,而 RNA 聚合酶 II 是 HIV-1 原病毒转录所必需的。AFF1/4 是一种支架蛋白,它招募 SEC 的其他组件并根据其 THD 结构域(具有处理区域二聚化结构域的 TPRL)形成二聚体。在这里,我们以 2.4 Å 的分辨率报告了人类 AFF4-THD 的晶体结构。一个 AFF4-THD 的 α4、α5 和 α6 介导二聚体的形成,并与 AFF-THD 二聚体中第二个分子的等效部分紧密堆积。诱变分析表明,AFF4 的 Phe1014 或 Tyr1096 单一突变为丙氨酸会损害 AFF4 二聚体的形成。此外,反式激活实验还表明AFF4的Phe1014和Tyr1096对于AFF4的反式激活活性至关重要。有趣的是,AFF1中相应的残基Phe1063和Tyr1145对HIV-1原病毒的反式激活有影响。然而,AFF1/4 的此类突变对 AFF1/4 与 SEC 其他亚基的相互作用没有影响。总之,我们的数据表明 AFF1/4 的二聚化对于 HIV-1 原病毒的反式激活至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号