Nature Medicine ( IF 58.7 ) Pub Date : 2020-02-17 , DOI: 10.1038/s41591-020-0753-3 Tyson J Moyer 1, 2 , Yu Kato 2, 3 , Wuhbet Abraham 1 , Jason Y H Chang 1 , Daniel W Kulp 2, 4, 5 , Nicki Watson 6 , Hannah L Turner 2, 5, 7 , Sergey Menis 2, 5 , Robert K Abbott 2, 3 , Jinal N Bhiman 2, 5, 8, 9 , Mariane B Melo 1, 2, 9 , Hayley A Simon 3 , Sara Herrera-De la Mata 3 , Shu Liang 3 , Gregory Seumois 3 , Yash Agarwal 1, 10 , Na Li 1 , Dennis R Burton 2, 5, 8, 9 , Andrew B Ward 2, 5, 7 , William R Schief 2, 5, 8, 9 , Shane Crotty 2, 3, 11 , Darrell J Irvine 1, 2, 9, 10, 12, 13
|
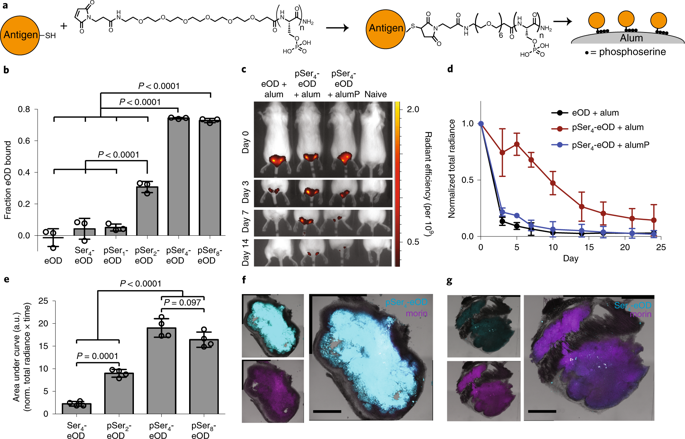
Adjuvants are central to the efficacy of subunit vaccines. Aluminum hydroxide (alum) is the most commonly used vaccine adjuvant, yet its adjuvanticity is often weak and mechanisms of triggering antibody responses remain poorly understood. We demonstrate that site-specific modification of immunogens with short peptides composed of repeating phosphoserine (pSer) residues enhances binding to alum and prolongs immunogen bioavailability. The pSer-modified immunogens formulated in alum elicited greatly increased germinal center, antibody, neutralizing antibody, memory and long-lived plasma cell responses compared to conventional alum-adsorbed immunogens. Mechanistically, pSer-immunogen:alum complexes form nanoparticles that traffic to lymph nodes and trigger B cell activation through multivalent and oriented antigen display. Direct uptake of antigen-decorated alum particles by B cells upregulated antigen processing and presentation pathways, further enhancing B cell activation. These data provide insights into mechanisms of action of alum and introduce a readily translatable approach to significantly improve humoral immunity to subunit vaccines using a clinical adjuvant.
中文翻译:

工程免疫原与明矾佐剂结合可增强体液免疫
佐剂是亚单位疫苗效力的核心。氢氧化铝(明矾)是最常用的疫苗佐剂,但其佐剂性通常很弱,引发抗体反应的机制仍然知之甚少。我们证明用由重复磷酸丝氨酸 (pSer) 残基组成的短肽对免疫原进行位点特异性修饰可增强与明矾的结合并延长免疫原的生物利用度。与传统的明矾吸附免疫原相比,在明矾中配制的 pSer 修饰的免疫原大大增加了生发中心、抗体、中和抗体、记忆和长寿命浆细胞反应。从机制上讲,pSer-免疫原:明矾复合物形成纳米颗粒,该纳米颗粒可运输至淋巴结并通过多价和定向抗原展示触发 B 细胞活化。B细胞直接摄取抗原修饰的明矾颗粒上调抗原加工和呈递途径,进一步增强B细胞活化。这些数据提供了对明矾作用机制的见解,并引入了一种易于转化的方法,以使用临床佐剂显着提高对亚单位疫苗的体液免疫。











































 京公网安备 11010802027423号
京公网安备 11010802027423号