Nature Chemical Biology ( IF 12.9 ) Pub Date : 2020-02-17 , DOI: 10.1038/s41589-020-0476-2 Arne Matthews 1 , Raspudin Saleem-Batcha 1 , Jacob N Sanders 2 , Frederick Stull 3 , K N Houk 2 , Robin Teufel 1
|
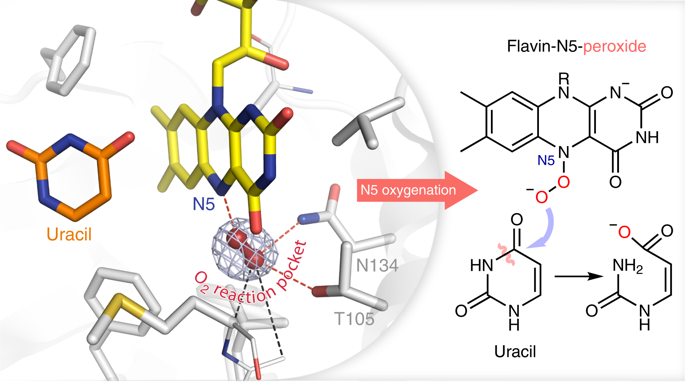
One of the hallmark reactions catalyzed by flavin-dependent enzymes is the incorporation of an oxygen atom derived from dioxygen into organic substrates. For many decades, these flavin monooxygenases were assumed to use exclusively the flavin-C4a-(hydro)peroxide as their oxygen-transferring intermediate. We demonstrate that flavoenzymes may instead employ a flavin-N5-peroxide as a soft α-nucleophile for catalysis, which enables chemistry not accessible to canonical monooxygenases. This includes, for example, the redox-neutral cleavage of carbon-hetero bonds or the dehalogenation of inert environmental pollutants via atypical oxygenations. We furthermore identify a shared structural motif for dioxygen activation and N5-functionalization, suggesting a conserved pathway that may be operative in numerous characterized and uncharacterized flavoenzymes from diverse organisms. Our findings show that overlooked flavin-N5-oxygen adducts are more widespread and may facilitate versatile chemistry, thus upending the notion that flavin monooxygenases exclusively function as nature’s equivalents to organic peroxides in synthetic chemistry.
中文翻译:

氨基过氧化物加合物扩展了黄素单加氧酶的催化库
黄素依赖性酶催化的标志性反应之一是将衍生自分子氧的氧原子结合到有机底物中。几十年来,这些黄素单加氧酶被认为只使用黄素-C4a-(氢)过氧化物作为它们的氧转移中间体。我们证明了黄素酶可以替代使用黄素-N5-过氧化物作为催化的软α-亲核试剂,这使得经典单加氧酶无法获得化学。这包括,例如,碳杂键的氧化还原中性裂解或惰性环境污染物通过非典型氧化作用的脱卤。我们进一步确定了分子氧激活和 N5 功能化的共享结构基序,表明可能在来自不同生物的许多特征和未特征的黄素酶中起作用的保守途径。我们的研究结果表明,被忽视的黄素-N5-氧加合物更为普遍,并可能促进多功能化学,从而颠覆了黄素单加氧酶在合成化学中仅作为有机过氧化物的天然等效物的观点。










































 京公网安备 11010802027423号
京公网安备 11010802027423号