The Lancet HIV ( IF 12.8 ) Pub Date : 2020-02-17 , DOI: 10.1016/s2352-3018(20)30001-1 Kathryn E Stephenson 1 , Frank Wegmann 2 , Frank Tomaka 3 , Stephen R Walsh 4 , C Sabrina Tan 4 , Ludo Lavreys 3 , Jessica L Ansel 4 , Diane G Kanjilal 4 , Kate Jaegle 4 , Joseph P Nkolola 4 , Lauren Peter 4 , Rachel Fogel 4 , Connor Bradshaw 4 , Anna Tyler 4 , Tatenda Makoni 4 , Lisa Howe 4 , Darla Quijada 4 , Abishek Chandrashekar 4 , Esther A Bondzie 4 , Erica N Borducchi 4 , Katherine E Yanosick 4 , Jenny Hendriks 2 , Steven Nijs 2 , Carla Truyers 2 , Jeroen Tolboom 2 , Roland C Zahn 2 , Michael S Seaman 4 , Galit Alter 5 , Daniel J Stieh 2 , Maria Grazia Pau 2 , Hanneke Schuitemaker 2 , Dan H Barouch 1
|
Background
Current efficacy studies of a mosaic HIV-1 prophylactic vaccine require four vaccination visits over one year, which is a complex regimen that could prove challenging for vaccine delivery at the community level, both for recipients and clinics. In this study, we evaluated the safety, tolerability, and immunogenicity of shorter, simpler regimens of trivalent Ad26.Mos.HIV expressing mosaic HIV-1 Env/Gag/Pol antigens combined with aluminium phosphate-adjuvanted clade C gp140 protein.
Methods
We did this randomised, double-blind, placebo-controlled phase 1 trial (IPCAVD010/HPX1002) at Beth Israel Deaconess Medical Center in Boston, MA, USA. We included healthy, HIV-uninfected participants (aged 18–50 years) who were considered at low risk for HIV infection and had not received any vaccines in the 14 days before study commencement. We randomly assigned participants via a computer-generated randomisation schedule and interactive web response system to one of three study groups (1:1:1) testing different regimens of trivalent Ad26.Mos.HIV (5 × 1010 viral particles per 0·5 mL) combined with 250 μg adjuvanted clade C gp140 protein. They were then assigned to treatment or placebo subgroups (5:1) within each of the three main groups. Participants and investigators were masked to treatment allocation until the end of the follow-up period. Group 1 received Ad26.Mos.HIV alone at weeks 0 and 12 and Ad26.Mos.HIV plus adjuvanted gp140 at weeks 24 and 48. Group 2 received Ad26.Mos.HIV plus adjuvanted gp140 at weeks 0, 12, and 24. Group 3 received Ad26.Mos.HIV alone at week 0 and Ad26.Mos.HIV plus adjuvanted gp140 at weeks 8 and 24. Participants in the control group received 0·5 mL of 0·9% saline. All study interventions were administered intramuscularly. The primary endpoints were Env-specific binding antibody responses at weeks 28, 52, and 72 and safety and tolerability of the vaccine regimens for 28 days after the injection. All participants who received at least one vaccine dose or placebo were included in the safety analysis; immunogenicity was analysed using the per-protocol population. The IPCAVD010/HPX1002 trial is registered with ClinicalTrials.gov, NCT02685020. We also did a parallel preclinical study in rhesus monkeys to test the protective efficacy of the shortened group 3 regimen.
Findings
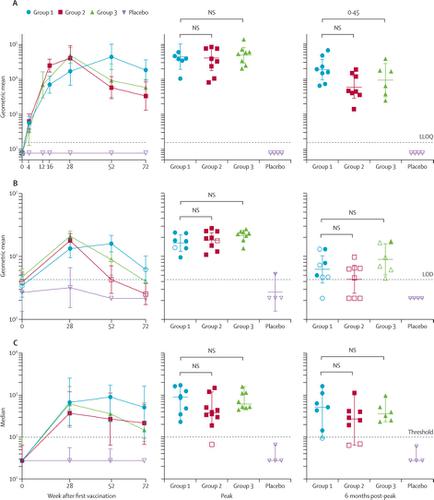
Between March 7, 2016, and Aug 19, 2016, we randomly assigned 36 participants to receive at least one dose of study vaccine or placebo, ten to each vaccine group and two to the corresponding placebo group. 30 (83%) participants completed the full study, and six (17%) discontinued it prematurely because of loss to follow-up, withdrawal of consent, investigator decision, and an unrelated death from a motor vehicle accident. The two shortened regimens elicited comparable antibody titres against autologous clade C Env at peak immunity to the longer, 12-month regimen: geometric mean titre (GMT) 41 007 (95% CI 17 959–93 636) for group 2 and 49 243 (29 346–82 630) for group 3 at week 28 compared with 44 590 (19 345–102 781) for group 1 at week 52). Antibody responses remained increased (GMT >5000) in groups 2 and 3 at week 52 but were highest in group 1 at week 72. Antibody-dependent cellular phagocytosis, Env-specific IgG3, tier 1A neutralising activity, and broad cellular immune responses were detected in all groups. All vaccine regimens were well tolerated. Mild-to-moderate pain or tenderness at the injection site was the most commonly reported solicited local adverse event, reported by 28 vaccine recipients (93%) and two placebo recipients (33%). Grade 3 solicited systemic adverse events were reported by eight (27%) vaccine recipients and no placebo recipients; the most commonly reported grade 3 systemic symptoms were fatigue, myalgia, and chills. The shortened group 3 regimen induced comparable peak immune responses in 30 rhesus monkeys as in humans and resulted in an 83% (95% CI 38·7–95, p=0·004 log-rank test) reduction in per-exposure acquisition risk after six intrarectal challenges with SHIV-SF162P3 at week 54, more than 6 months after final vaccination.
Interpretation
Short, 6-month regimens of a mosaic HIV-1 prophylactic vaccine elicited robust HIV-specific immune responses that were similar to responses elicited by a longer, 12-month schedule. Preclinical data showed partial protective efficacy of one of the short vaccine regimens in rhesus monkeys. Further clinical studies are required to test the suitability of the shortened vaccine regimens in humans. Such shortened regimens would be valuable to increase vaccine delivery at the community level, particularly in resource-limited settings.
Funding
Ragon Institute (Massachusetts General Hospital, Massachusetts Institute of Technology, and Harvard University; Cambridge, MA, USA) and Janssen Vaccines & Prevention (Leiden, Netherlands).
中文翻译:

缩短的镶嵌HIV-1疫苗接种时间表的比较:一项随机,双盲,安慰剂对照的1期临床试验(IPCAVD010 / HPX1002)和恒河猴的临床前研究(NHP 17-22)。
背景
目前对镶嵌HIV-1预防性疫苗的功效研究需要在一年内进行四次疫苗接种,这是一个复杂的方案,可能会对社区一级的接受者和诊所的疫苗交付带来挑战。在这项研究中,我们评估了表达镶嵌HIV-1 Env / Gag / Pol抗原与磷酸铝佐剂C gp140蛋白结合的三价Ad26.Mos.HIV较短,更简单方案的安全性,耐受性和免疫原性。
方法
我们在美国马萨诸塞州波士顿的贝斯以色列女执事医疗中心进行了这项随机,双盲,安慰剂对照的1期临床试验(IPCAVD010 / HPX1002)。我们纳入了健康的,未感染HIV的参与者(年龄18至50岁),这些参与者被认为感染HIV的风险较低,并且在研究开始前的14天内未接种任何疫苗。我们通过计算机生成的随机对照表和交互式网络响应系统,将参与者随机分配到三个研究组(1:1:1)中,以测试三价Ad26.Mos.HIV(5×10 10病毒颗粒(每0·5 mL)与250μg佐剂进化枝C gp140蛋白结合。然后将他们分配到三个主要组别中的治疗或安慰剂亚组(5:1)。参加者和研究者被掩盖了治疗分配,直到随访期结束。第1组在第0和12周时分别接受Ad26.Mos.HIV,在第24和48周时接受Ad26.Mos.HIV加佐剂的gp140。第2组在第0、12和24周时接受Ad26.Mos.HIV加佐剂的gp140。 3名患者在第0周时单独接受Ad26.Mos.HIV,在第8和24周时接受Ad26.Mos.HIV加佐剂gp140。对照组参与者接受0·5 mL的0·9%生理盐水。所有研究干预均通过肌肉注射进行。主要终点为在第28、52周的Env特异性结合抗体反应,72和注射后28天的疫苗接种方案的安全性和耐受性。所有接受至少一种疫苗剂量或安慰剂的参与者都包括在安全性分析中;使用按协议人群分析免疫原性。IPCAVD010 / HPX1002试用版已在ClinicalTrials.gov注册为NCT02685020。我们还对恒河猴进行了平行的临床前研究,以测试缩短的第3组方案的保护作用。
发现
在2016年3月7日至2016年8月19日之间,我们随机分配了36名参与者接受至少一剂研究疫苗或安慰剂,每个疫苗组十只,相应的安慰剂组两剂。30名(83%)的参与者完成了整个研究,而六名(17%)的参与者由于失去随访,撤回同意,调查人员的决定以及与机动车事故无关的死亡而过早终止研究。两种缩短的治疗方案在对更长的12个月治疗方案的免疫力达到峰值时,对自体进化枝C Env产生了相似的抗体效价:第2组和49 243(GMT)41 007(95%CI 17 959–93 636)第28周的第3组为29 346–82 630),而第52周的第1组为44 590(19 345–102 781)。抗体反应仍然增强(GMT> 在第52周的第2组和第3组中,有5000例),但在第72周的组1中是最高的。在所有组中均检测到了抗体依赖性细胞吞噬作用,Env特异性IgG3、1A级中和活性和广泛的细胞免疫应答。所有疫苗方案均耐受良好。注射部位轻至中度的疼痛或压痛是最常报告的局部不良事件,有28位疫苗接种者(93%)和2位安慰剂接受者(33%)报告。8名(27%)疫苗接种者报告了3级全身性不良事件,无安慰剂接种者;最常见的3级全身症状是疲劳,肌痛和发冷。缩短的第3组治疗方案可在30只恒河猴中诱导出与人类相当的峰值免疫反应,结果是83%(95%CI 38·7–95,
解释
镶嵌HIV-1预防性疫苗的短短6个月疗程可产生强有力的HIV特异性免疫反应,类似于更长的12个月的治疗方案所产生的反应。临床前数据显示,一种短疫苗接种方案对恒河猴有部分保护作用。需要进一步的临床研究来测试缩短的疫苗接种方案在人类中的适用性。这种缩短的疗程对于增加社区一级的疫苗交付非常有价值,特别是在资源有限的环境中。
资金
Ragon研究所(麻省理工学院麻萨诸塞州总医院,哈佛大学;美国马萨诸塞州剑桥市)和Janssen疫苗与预防所(荷兰莱顿)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号