Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mitochondrial division inhibitor 1 disrupts oligodendrocyte Ca2+ homeostasis and mitochondrial function.
Glia ( IF 5.4 ) Pub Date : 2020-02-14 , DOI: 10.1002/glia.23802 Asier Ruiz 1 , Tania Quintela-López 1, 2 , María V Sánchez-Gómez 1 , Adhara Gaminde-Blasco 1 , Elena Alberdi 1 , Carlos Matute 1
Glia ( IF 5.4 ) Pub Date : 2020-02-14 , DOI: 10.1002/glia.23802 Asier Ruiz 1 , Tania Quintela-López 1, 2 , María V Sánchez-Gómez 1 , Adhara Gaminde-Blasco 1 , Elena Alberdi 1 , Carlos Matute 1
Affiliation
|
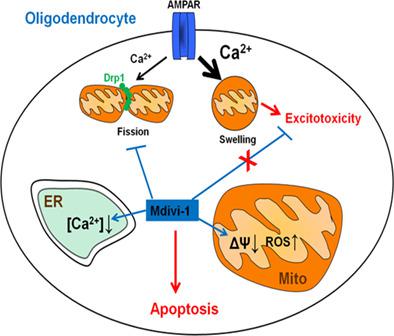
Mitochondrial fission mediated by cytosolic dynamin related protein 1 (Drp1) is essential for mitochondrial quality control but may contribute to apoptosis as well. Blockade of Drp1 with mitochondrial division inhibitor 1 (mdivi‐1) provides neuroprotection in several models of neurodegeneration and cerebral ischemia and has emerged as a promising therapeutic drug. In oligodendrocytes, overactivation of AMPA‐type ionotropic glutamate receptors (AMPARs) induces intracellular Ca2+ overload and excitotoxic death that contributes to demyelinating diseases. Mitochondria are key to Ca2+ homeostasis, however it is unclear how it is disrupted during oligodendroglial excitotoxicity. In the current study, we have analyzed mitochondrial dynamics during AMPAR activation and the effects of mdivi‐1 on excitotoxicity in optic nerve‐derived oligodendrocytes. Sublethal AMPAR activation triggered Drp1‐dependent mitochondrial fission, whereas toxic AMPAR activation produced Drp1‐independent mitochondrial swelling. Accordingly, mdivi‐1 efficiently inhibited Drp1‐mediated mitochondrial fission and did not prevent oligodendrocyte excitotoxicity. Unexpectedly, mdivi‐1 also induced mitochondrial depolarization, ER Ca2+ depletion and modulation of AMPA‐induced Ca2+ signaling. These off‐target effects of mdivi‐1 sensitized oligodendrocytes to excitotoxicity and ER stress and eventually produced oxidative stress and apoptosis. Interestingly, in cultured astrocytes mdivi‐1 induced nondetrimental mitochondrial depolarization and oxidative stress that did not cause toxicity or sensitization to apoptotic stimuli. In summary, our results provide evidence of Drp1‐mediated mitochondrial fission during activation of ionotropic glutamate receptors in oligodendrocytes, and uncover a deleterious and Drp1‐independent effect of mdivi‐1 on mitochondrial and ER function in these cells. These off‐target effects of mdivi‐1 limit its therapeutic potential and should be taken into account in clinical studies.
中文翻译:

线粒体分裂抑制剂 1 破坏少突胶质细胞 Ca2+ 稳态和线粒体功能。
由胞质动力蛋白相关蛋白 1 (Drp1) 介导的线粒体裂变对于线粒体质量控制至关重要,但也可能导致细胞凋亡。用线粒体分裂抑制剂 1 (mdivi-1) 阻断 Drp1 在几种神经变性和脑缺血模型中提供神经保护,并已成为一种有前途的治疗药物。在少突胶质细胞中,AMPA 型离子型谷氨酸受体 (AMPAR) 的过度激活会诱导细胞内 Ca 2+超载和兴奋性毒性死亡,从而导致脱髓鞘疾病。线粒体是 Ca 2+ 的关键体内平衡,但尚不清楚它是如何在少突胶质细胞兴奋性毒性期间被破坏的。在目前的研究中,我们分析了 AMPAR 激活过程中的线粒体动力学以及 mdivi-1 对视神经源性少突胶质细胞兴奋性毒性的影响。亚致死的 AMPAR 激活触发了依赖 Drp1 的线粒体裂变,而有毒的 AMPAR 激活产生了依赖于 Drp1 的线粒体肿胀。因此,mdivi-1 有效地抑制了 Drp1 介导的线粒体裂变,并不能阻止少突胶质细胞的兴奋性毒性。出乎意料的是,mdivi-1 还诱导线粒体去极化、ER Ca 2+耗竭和 AMPA 诱导的 Ca 2+调节信号。mdivi-1 的这些脱靶效应使少突胶质细胞对兴奋性毒性和内质网应激敏感,并最终产生氧化应激和细胞凋亡。有趣的是,在培养的星形胶质细胞中,mdivi-1 诱导了无害的线粒体去极化和氧化应激,不会引起毒性或对凋亡刺激物的敏感性。总之,我们的结果提供了在少突胶质细胞中的离子型谷氨酸受体激活过程中 Drp1 介导的线粒体裂变的证据,并揭示了 mdivi-1 对这些细胞中的线粒体和 ER 功能的有害且不依赖 Drp1 的影响。mdivi-1 的这些脱靶效应限制了其治疗潜力,应在临床研究中予以考虑。
更新日期:2020-02-14
中文翻译:

线粒体分裂抑制剂 1 破坏少突胶质细胞 Ca2+ 稳态和线粒体功能。
由胞质动力蛋白相关蛋白 1 (Drp1) 介导的线粒体裂变对于线粒体质量控制至关重要,但也可能导致细胞凋亡。用线粒体分裂抑制剂 1 (mdivi-1) 阻断 Drp1 在几种神经变性和脑缺血模型中提供神经保护,并已成为一种有前途的治疗药物。在少突胶质细胞中,AMPA 型离子型谷氨酸受体 (AMPAR) 的过度激活会诱导细胞内 Ca 2+超载和兴奋性毒性死亡,从而导致脱髓鞘疾病。线粒体是 Ca 2+ 的关键体内平衡,但尚不清楚它是如何在少突胶质细胞兴奋性毒性期间被破坏的。在目前的研究中,我们分析了 AMPAR 激活过程中的线粒体动力学以及 mdivi-1 对视神经源性少突胶质细胞兴奋性毒性的影响。亚致死的 AMPAR 激活触发了依赖 Drp1 的线粒体裂变,而有毒的 AMPAR 激活产生了依赖于 Drp1 的线粒体肿胀。因此,mdivi-1 有效地抑制了 Drp1 介导的线粒体裂变,并不能阻止少突胶质细胞的兴奋性毒性。出乎意料的是,mdivi-1 还诱导线粒体去极化、ER Ca 2+耗竭和 AMPA 诱导的 Ca 2+调节信号。mdivi-1 的这些脱靶效应使少突胶质细胞对兴奋性毒性和内质网应激敏感,并最终产生氧化应激和细胞凋亡。有趣的是,在培养的星形胶质细胞中,mdivi-1 诱导了无害的线粒体去极化和氧化应激,不会引起毒性或对凋亡刺激物的敏感性。总之,我们的结果提供了在少突胶质细胞中的离子型谷氨酸受体激活过程中 Drp1 介导的线粒体裂变的证据,并揭示了 mdivi-1 对这些细胞中的线粒体和 ER 功能的有害且不依赖 Drp1 的影响。mdivi-1 的这些脱靶效应限制了其治疗潜力,应在临床研究中予以考虑。











































 京公网安备 11010802027423号
京公网安备 11010802027423号