当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-catalyzed enantioselective conjugate addition of organometallic reagents to challenging Michael acceptors
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-02-17 , DOI: 10.3762/bjoc.16.24 Delphine Pichon , Jennifer Morvan , Christophe Crévisy , Marc Mauduit
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-02-17 , DOI: 10.3762/bjoc.16.24 Delphine Pichon , Jennifer Morvan , Christophe Crévisy , Marc Mauduit
|
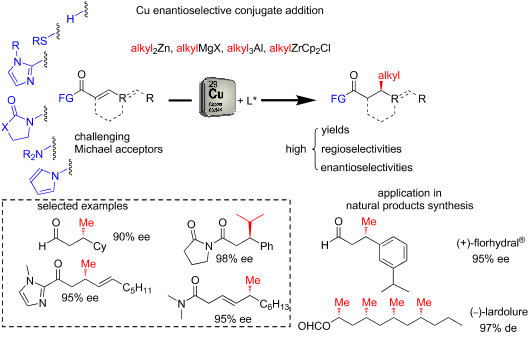
The copper-catalyzed enantioselective conjugate addition (ECA) of organometallic nucleophiles to electron-deficient alkenes (Michael acceptors) represents an efficient and attractive methodology for providing a wide range of relevant chiral molecules. In order to increase the attractiveness of this useful catalytic transformation, some Michael acceptors bearing challenging electron-deficient functions (i.e., aldehydes, thioesters, acylimidazoles, N-acyloxazolidinones, N-acylpyrrolidinones, amides, N-acylpyrroles) were recently investigated. Remarkably, only a few chiral copper-based catalytic systems have successfully achieved the conjugate addition of different organometallic reagents to these challenging Michael acceptors, with excellent regio- and enantioselectivity. Furthermore, thanks to their easy derivatization, the resulting chiral conjugated products could be converted into various natural products. The aim of this tutorial review is to summarize recent advances accomplished in this stimulating field.
中文翻译:

有机铜试剂的铜催化对映选择性共轭加成反应
有机金属亲核试剂向缺电子烯烃(Michael受体)的铜催化对映选择性共轭加成(ECA)代表了一种有效且有吸引力的方法,可提供各种相关的手性分子。为了增加这种有用的催化转化的吸引力,一些具有挑战性的电子缺陷功能的迈克尔受体(例如,醛,硫酯,酰基咪唑,N-酰基恶唑烷酮,N-酰基吡咯烷酮,酰胺,N-酰基吡咯)。值得注意的是,只有极少数的手性铜基催化体系成功地将不同的有机金属试剂共轭添加到这些具有挑战性的Michael受体上,并具有出色的区域和对映选择性。此外,由于它们易于衍生,所得到的手性共轭产物可以转化成各种天然产物。本教程复习的目的是总结在这个刺激领域中取得的最新进展。
更新日期:2020-02-18
中文翻译:

有机铜试剂的铜催化对映选择性共轭加成反应
有机金属亲核试剂向缺电子烯烃(Michael受体)的铜催化对映选择性共轭加成(ECA)代表了一种有效且有吸引力的方法,可提供各种相关的手性分子。为了增加这种有用的催化转化的吸引力,一些具有挑战性的电子缺陷功能的迈克尔受体(例如,醛,硫酯,酰基咪唑,N-酰基恶唑烷酮,N-酰基吡咯烷酮,酰胺,N-酰基吡咯)。值得注意的是,只有极少数的手性铜基催化体系成功地将不同的有机金属试剂共轭添加到这些具有挑战性的Michael受体上,并具有出色的区域和对映选择性。此外,由于它们易于衍生,所得到的手性共轭产物可以转化成各种天然产物。本教程复习的目的是总结在这个刺激领域中取得的最新进展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号