当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modulation of Coiled-Coil Binding Strength and Fusogenicity through Peptide Stapling.
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-02-14 , DOI: 10.1021/acs.bioconjchem.0c00009 Niek S A Crone 1 , Alexander Kros 1 , Aimee L Boyle 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-02-14 , DOI: 10.1021/acs.bioconjchem.0c00009 Niek S A Crone 1 , Alexander Kros 1 , Aimee L Boyle 1
Affiliation
|
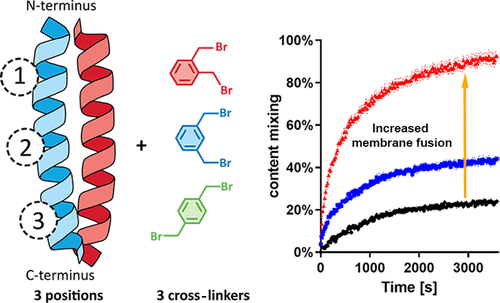
Peptide stapling is a technique which has been widely employed to constrain the conformation of peptides. One of the effects of such a constraint can be to modulate the interaction of the peptide with a binding partner. Here, a cysteine bis-alkylation stapling technique was applied to generate structurally isomeric peptide variants of a heterodimeric coiled-coil forming peptide. These stapled variants differed in the position and size of the formed macrocycle. C-terminal stapling showed the most significant changes in peptide structure and stability, with calorimetric binding analysis showing a significant reduction of binding entropy for stapled variants. This entropy reduction was dependent on cross-linker size and was accompanied by a change in binding enthalpy, illustrating the effects of preorganization. The stapled peptide, along with its binding partner, were subsequently employed as fusogens in a liposome model system. An increase in both lipid- and content-mixing was observed for one of the stapled peptide variants: this increased fusogenicity was attributed to increased coiled-coil binding but not to membrane affinity, an interaction theorized to be a primary driving force in this fusion system.
中文翻译:

通过肽装订调节卷曲螺旋结合强度和融合性。
肽装订是一种广泛用于限制肽构象的技术。这种限制的作用之一是调节肽与结合配偶体的相互作用。在这里,应用半胱氨酸双烷基化装订技术来生成异二聚体卷曲螺旋形成肽的结构异构肽变体。这些钉合变体在形成的大环的位置和大小方面有所不同。 C 端装订显示出肽结构和稳定性的最显着变化,量热结合分析显示装订变体的结合熵显着降低。这种熵的减少取决于交联剂的大小,并伴随着结合焓的变化,说明了预组织的影响。随后,钉合肽及其结合伴侣被用作脂质体模型系统中的融合剂。对于一种钉合肽变体,观察到脂质和内容物混合的增加:这种融合性的增加归因于卷曲螺旋结合的增加,而不是膜亲和力,理论上这种相互作用是该融合系统的主要驱动力。
更新日期:2020-02-28
中文翻译:

通过肽装订调节卷曲螺旋结合强度和融合性。
肽装订是一种广泛用于限制肽构象的技术。这种限制的作用之一是调节肽与结合配偶体的相互作用。在这里,应用半胱氨酸双烷基化装订技术来生成异二聚体卷曲螺旋形成肽的结构异构肽变体。这些钉合变体在形成的大环的位置和大小方面有所不同。 C 端装订显示出肽结构和稳定性的最显着变化,量热结合分析显示装订变体的结合熵显着降低。这种熵的减少取决于交联剂的大小,并伴随着结合焓的变化,说明了预组织的影响。随后,钉合肽及其结合伴侣被用作脂质体模型系统中的融合剂。对于一种钉合肽变体,观察到脂质和内容物混合的增加:这种融合性的增加归因于卷曲螺旋结合的增加,而不是膜亲和力,理论上这种相互作用是该融合系统的主要驱动力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号