当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antimony Sorption to Goethite: Effects of Fe(II)-Catalyzed Recrystallization
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-02-27 , DOI: 10.1021/acsearthspacechem.0c00013 Edward D. Burton 1 , Kerstin Hockmann 2 , Niloofar Karimian 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-02-27 , DOI: 10.1021/acsearthspacechem.0c00013 Edward D. Burton 1 , Kerstin Hockmann 2 , Niloofar Karimian 1
Affiliation
|
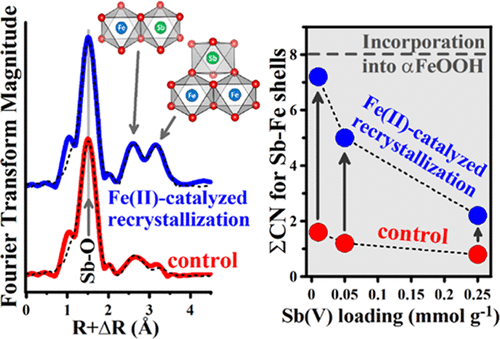
The environmental mobility and bioavailability of antimony (Sb) are strongly influenced by sorption to Fe(III) oxide minerals, such as goethite (αFeOOH). Exposure to aqueous Fe(II), as occurs, for example, under reducing conditions in soils and sediments, catalyzes the recrystallization of goethite. Herein, we examine, for the first time, the effect of Fe(II)-catalyzed goethite recrystallization on the sorption of co-associated Sb(V). The use of an enriched aqueous 57Fe(II) tracer provided direct evidence of Fe(II)-catalyzed goethite recrystallization and revealed lower levels of recrystallization at higher Sb(V) loadings. Goethite recrystallization caused increases in the total amount of sorbed Sb along with decreases in the percentage of sorbed Sb that was surface-bound (based on PO43– extractions). Extended X-ray absorption fine structure (EXAFS) spectroscopy at the Sb K-edge shows that goethite recrystallization led to increases in the coordination number (CN) for both edge-sharing and double-corner-sharing linkages between Sb(V)–O and Fe(III)–O octahedra. The CN increases are consistent with partial structural Sb(V) incorporation into goethite, with the degree of incorporation increasing with the extent of recrystallization. Overall, the results indicate that Fe(II)-catalyzed recrystallization of goethite caused a shift from Sb(V) adsorption at the goethite surface to partial Sb(V) incorporation into the goethite structure [via heterovalent substitution for up to ∼1.4 mol % Fe(III)]. These results necessitate a re-evaluation of current concepts regarding the sorption of Sb to goethite. In particular, goethite should be considered as a dynamic phase whose solid structure (not just the reactive surface) may be available for Sb(V) sorption via structural incorporation during Fe(II)-catalyzed recrystallization.
中文翻译:

针铁矿对锑的吸附:Fe(II)催化重结晶的影响
锑(Sb)的环境迁移率和生物利用度受吸附于针铁矿(αFeOOH)的Fe(III)氧化物矿物质的强烈影响。暴露于Fe(II)水溶液(例如在还原条件下的土壤和沉积物中发生)会催化针铁矿的重结晶。在这里,我们首次检查了铁(II)催化针铁矿重结晶对共缔合Sb(V)吸附的影响。使用富集的57 Fe(II)示踪剂水溶液可提供Fe(II)催化针铁矿重结晶的直接证据,并显示在较高的Sb(V)负载下较低的重结晶水平。针铁矿重结晶导致吸附的Sb总量增加以及表面结合的吸附Sb百分比降低(基于PO 43 –提取物)。Sb K边缘的扩展X射线吸收精细结构(EXAFS)光谱显示,针铁矿重结晶导致Sb(V)-O之间的边缘共享和双角共享键的配位数(CN)增加和Fe(III)–O八面体。CN的增加与向针铁矿中掺入部分结构性Sb(V)一致,掺入程度随重结晶程度的增加而增加。总体而言,结果表明,Fe(II)催化的针铁矿重结晶导致从针铁矿表面的Sb(V)吸附转变为部分Sb(V)掺入针铁矿结构[通过异价取代达到约1.4 mol% Fe(III)]。这些结果需要重新评估有关Sb吸附到针铁矿中的当前概念。尤其是,
更新日期:2020-02-28
中文翻译:

针铁矿对锑的吸附:Fe(II)催化重结晶的影响
锑(Sb)的环境迁移率和生物利用度受吸附于针铁矿(αFeOOH)的Fe(III)氧化物矿物质的强烈影响。暴露于Fe(II)水溶液(例如在还原条件下的土壤和沉积物中发生)会催化针铁矿的重结晶。在这里,我们首次检查了铁(II)催化针铁矿重结晶对共缔合Sb(V)吸附的影响。使用富集的57 Fe(II)示踪剂水溶液可提供Fe(II)催化针铁矿重结晶的直接证据,并显示在较高的Sb(V)负载下较低的重结晶水平。针铁矿重结晶导致吸附的Sb总量增加以及表面结合的吸附Sb百分比降低(基于PO 43 –提取物)。Sb K边缘的扩展X射线吸收精细结构(EXAFS)光谱显示,针铁矿重结晶导致Sb(V)-O之间的边缘共享和双角共享键的配位数(CN)增加和Fe(III)–O八面体。CN的增加与向针铁矿中掺入部分结构性Sb(V)一致,掺入程度随重结晶程度的增加而增加。总体而言,结果表明,Fe(II)催化的针铁矿重结晶导致从针铁矿表面的Sb(V)吸附转变为部分Sb(V)掺入针铁矿结构[通过异价取代达到约1.4 mol% Fe(III)]。这些结果需要重新评估有关Sb吸附到针铁矿中的当前概念。尤其是,











































 京公网安备 11010802027423号
京公网安备 11010802027423号