当前位置:
X-MOL 学术
›
STEM CELLS
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functional dosing of mesenchymal stromal cell-derived extracellular vesicles for the prevention of acute graft-versus-host-disease
STEM CELLS ( IF 4.0 ) Pub Date : 2020-02-17 , DOI: 10.1002/stem.3160 Giada Dal Collo 1 , Annalisa Adamo 1 , Alessandro Gatti 1 , Edoardo Tamellini 1 , Riccardo Bazzoni 1 , Paul Takam Kamga 1, 2 , Cristina Tecchio 1 , Francesca Maria Quaglia 1 , Mauro Krampera 1
STEM CELLS ( IF 4.0 ) Pub Date : 2020-02-17 , DOI: 10.1002/stem.3160 Giada Dal Collo 1 , Annalisa Adamo 1 , Alessandro Gatti 1 , Edoardo Tamellini 1 , Riccardo Bazzoni 1 , Paul Takam Kamga 1, 2 , Cristina Tecchio 1 , Francesca Maria Quaglia 1 , Mauro Krampera 1
Affiliation
|
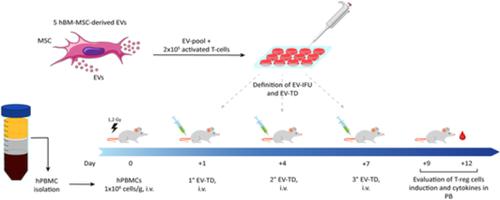
Graft‐vs‐host‐disease (GvHD) is currently the main complication of allogeneic hematopoietic stem cell transplantation. Mortality and morbidity rates are particularly high, especially in steroid‐refractory acute GvHD (aGvHD). Immune regulatory human bone marrow mesenchymal stromal cells (hMB‐MSCs) represent a therapeutic approach to address this issue. Unfortunately, their effect is hardly predictable in vivo due to several variables, that is, MSC tissue origin, concentration, dose number, administration route and timing, and inflammatory status of the recipient. Interestingly, human bone marrow MSC‐derived extracellular vesicles (hBM‐MSC‐EVs) display many of the hBM‐MSC immunoregulatory properties due to their content in paracrine factors that greatly varies according to the collection method. In this study, we focused on the immunological characterization of hBM‐MSC‐EVs on their capability of inducing regulatory T‐cells (T‐regs) both in vitro and in a xenograft mouse model of aGvHD. We correlated these data with the aGvHD incidence and degree following hBM‐MSC‐EV intravenous administration. Thus, we first quantified the EV immunomodulation in vitro in terms of EV immunomodulatory functional unit (EV‐IFU), that is, the lowest concentration of EVs leading in vitro to at least threefold increase of the T‐regs compared with controls. Second, we established the EV therapeutic dose in vivo (EV‐TD) corresponding to 10‐fold the in vitro EV‐IFU. According to this approach, we observed a significant improvement of both mouse survival and control of aGvHD onset and progression. This study confirms that EVs may represent an alternative to whole MSCs for aGvHD prevention, once the effective dose is reproducibly identified according to EV‐IFU and EV‐TD definition.
中文翻译:

用于预防急性移植物抗宿主病的间充质基质细胞衍生的细胞外囊泡的功能剂量
移植物抗宿主病(GvHD)是目前异基因造血干细胞移植的主要并发症。死亡率和发病率特别高,尤其是在类固醇难治性急性 GvHD (aGvHD) 中。免疫调节人骨髓间充质基质细胞 (hMB-MSCs) 代表了解决这一问题的一种治疗方法。不幸的是,由于几个变量,即 MSC 组织来源、浓度、剂量数、给药途径和时间以及受体的炎症状态,它们的作用在体内很难预测。有趣的是,人骨髓 MSC 衍生的细胞外囊泡 (hBM-MSC-EVs) 显示出许多 hBM-MSC 免疫调节特性,因为它们在旁分泌因子中的含量因收集方法而异。在这项研究中,我们专注于 hBM-MSC-EVs 在体外和 aGvHD 的异种移植小鼠模型中诱导调节性 T 细胞(T-regs)的能力的免疫学表征。我们将这些数据与 hBM-MSC-EV 静脉给药后的 aGvHD 发生率和程度相关联。因此,我们首先根据 EV 免疫调节功能单元 (EV-IFU) 量化了体外 EV 免疫调节,即与对照相比,在体外导致 T-regs 至少增加三倍的最低浓度的 EV。其次,我们建立了相当于体外 EV-IFU 10 倍的体内 EV 治疗剂量(EV-TD)。根据这种方法,我们观察到小鼠存活率和 aGvHD 发作和进展控制的显着改善。
更新日期:2020-02-17
中文翻译:

用于预防急性移植物抗宿主病的间充质基质细胞衍生的细胞外囊泡的功能剂量
移植物抗宿主病(GvHD)是目前异基因造血干细胞移植的主要并发症。死亡率和发病率特别高,尤其是在类固醇难治性急性 GvHD (aGvHD) 中。免疫调节人骨髓间充质基质细胞 (hMB-MSCs) 代表了解决这一问题的一种治疗方法。不幸的是,由于几个变量,即 MSC 组织来源、浓度、剂量数、给药途径和时间以及受体的炎症状态,它们的作用在体内很难预测。有趣的是,人骨髓 MSC 衍生的细胞外囊泡 (hBM-MSC-EVs) 显示出许多 hBM-MSC 免疫调节特性,因为它们在旁分泌因子中的含量因收集方法而异。在这项研究中,我们专注于 hBM-MSC-EVs 在体外和 aGvHD 的异种移植小鼠模型中诱导调节性 T 细胞(T-regs)的能力的免疫学表征。我们将这些数据与 hBM-MSC-EV 静脉给药后的 aGvHD 发生率和程度相关联。因此,我们首先根据 EV 免疫调节功能单元 (EV-IFU) 量化了体外 EV 免疫调节,即与对照相比,在体外导致 T-regs 至少增加三倍的最低浓度的 EV。其次,我们建立了相当于体外 EV-IFU 10 倍的体内 EV 治疗剂量(EV-TD)。根据这种方法,我们观察到小鼠存活率和 aGvHD 发作和进展控制的显着改善。











































 京公网安备 11010802027423号
京公网安备 11010802027423号