当前位置:
X-MOL 学术
›
Aging Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
TRPV1 activation alleviates cognitive and synaptic plasticity impairments through inhibiting AMPAR endocytosis in APP23/PS45 mouse model of Alzheimer's disease.
Aging Cell ( IF 8.0 ) Pub Date : 2020-02-14 , DOI: 10.1111/acel.13113 Yehong Du 1 , Min Fu 1 , Zhilin Huang 1 , Xin Tian 2 , Junjie Li 1 , Yayan Pang 1 , Weihong Song 1, 3 , Yu Tian Wang 1, 4 , Zhifang Dong 1
Aging Cell ( IF 8.0 ) Pub Date : 2020-02-14 , DOI: 10.1111/acel.13113 Yehong Du 1 , Min Fu 1 , Zhilin Huang 1 , Xin Tian 2 , Junjie Li 1 , Yayan Pang 1 , Weihong Song 1, 3 , Yu Tian Wang 1, 4 , Zhifang Dong 1
Affiliation
|
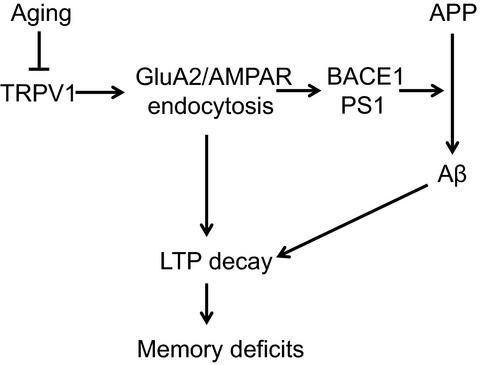
Alzheimer's disease (AD) is one of the most common causes of neurodegenerative diseases in the elderly. The accumulation of amyloid‐β (Aβ) peptides is one of the pathological hallmarks of AD and leads to the impairments of synaptic plasticity and cognitive function. The transient receptor potential vanilloid 1 (TRPV1), a nonselective cation channel, is involved in synaptic plasticity and memory. However, the role of TRPV1 in AD pathogenesis remains largely elusive. Here, we reported that the expression of TRPV1 was decreased in the brain of APP23/PS45 double transgenic AD model mice. Genetic upregulation of TRPV1 by adeno‐associated virus (AAV) inhibited the APP processing and Aβ deposition in AD model mice. Meanwhile, upregulation of TRPV1 ameliorated the deficits of hippocampal CA1 long‐term potentiation (LTP) and spatial learning and memory through inhibiting GluA2‐containing α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptor (AMPAR) endocytosis. Furthermore, pharmacological activation of TRPV1 by capsaicin (1 mg/kg, i.p.), an agonist of TRPV1, dramatically reversed the impairments of hippocampal CA1 LTP and spatial learning and memory in AD model mice. Taken together, these results indicate that TRPV1 activation effectively ameliorates cognitive and synaptic functions through inhibiting AMPAR endocytosis in AD model mice and could be a novel molecule for AD treatment.
中文翻译:

TRPV1激活通过抑制阿兹海默氏病APP23 / PS45小鼠模型中的AMPAR内吞作用减轻认知和突触可塑性损伤。
阿尔茨海默氏病(AD)是老年人神经退行性疾病的最常见原因之一。淀粉样β(Aβ)肽的积累是AD的病理标志之一,并导致突触可塑性和认知功能受损。瞬态受体电位香草酸1(TRPV1),非选择性阳离子通道,参与突触可塑性和记忆。但是,TRPV1在AD发病机理中的作用仍然难以捉摸。在这里,我们报道TRPV1的表达在APP23 / PS45双转基因AD模型小鼠的大脑中减少。腺相关病毒(AAV)对TRPV1的遗传上调抑制了AD模型小鼠的APP加工和Aβ沉积。与此同时,TRPV1的上调通过抑制含GluA2的α-氨基-3-羟基-5-甲基-4-异恶唑丙酸受体(AMPAR)的内吞作用,减轻了海马CA1长期增强(LTP)和空间学习与记忆的缺陷。此外,TRPV1的激动剂辣椒素(1 mg / kg,腹腔注射)对TRPV1的药理激活极大地逆转了AD模型小鼠的海马CA1 LTP损伤和空间学习与记忆。综上所述,这些结果表明TRPV1激活通过抑制AD模型小鼠中的AMPAR内吞作用有效地改善了认知和突触功能,并且可能是用于AD治疗的新分子。辣椒素(1 mg / kg,腹膜内)(TRPV1的激动剂)对TRPV1的药理激活极大地逆转了AD模型小鼠的海马CA1 LTP损伤和空间学习与记忆。综上所述,这些结果表明TRPV1激活通过抑制AD模型小鼠中的AMPAR内吞作用有效地改善了认知和突触功能,并且可能是用于AD治疗的新分子。辣椒素(1 mg / kg,腹膜内)(TRPV1的激动剂)对TRPV1的药理激活极大地逆转了AD模型小鼠的海马CA1 LTP损伤和空间学习与记忆。综上所述,这些结果表明TRPV1激活通过抑制AD模型小鼠中的AMPAR内吞作用有效地改善了认知和突触功能,并且可能是用于AD治疗的新分子。
更新日期:2020-02-14
中文翻译:

TRPV1激活通过抑制阿兹海默氏病APP23 / PS45小鼠模型中的AMPAR内吞作用减轻认知和突触可塑性损伤。
阿尔茨海默氏病(AD)是老年人神经退行性疾病的最常见原因之一。淀粉样β(Aβ)肽的积累是AD的病理标志之一,并导致突触可塑性和认知功能受损。瞬态受体电位香草酸1(TRPV1),非选择性阳离子通道,参与突触可塑性和记忆。但是,TRPV1在AD发病机理中的作用仍然难以捉摸。在这里,我们报道TRPV1的表达在APP23 / PS45双转基因AD模型小鼠的大脑中减少。腺相关病毒(AAV)对TRPV1的遗传上调抑制了AD模型小鼠的APP加工和Aβ沉积。与此同时,TRPV1的上调通过抑制含GluA2的α-氨基-3-羟基-5-甲基-4-异恶唑丙酸受体(AMPAR)的内吞作用,减轻了海马CA1长期增强(LTP)和空间学习与记忆的缺陷。此外,TRPV1的激动剂辣椒素(1 mg / kg,腹腔注射)对TRPV1的药理激活极大地逆转了AD模型小鼠的海马CA1 LTP损伤和空间学习与记忆。综上所述,这些结果表明TRPV1激活通过抑制AD模型小鼠中的AMPAR内吞作用有效地改善了认知和突触功能,并且可能是用于AD治疗的新分子。辣椒素(1 mg / kg,腹膜内)(TRPV1的激动剂)对TRPV1的药理激活极大地逆转了AD模型小鼠的海马CA1 LTP损伤和空间学习与记忆。综上所述,这些结果表明TRPV1激活通过抑制AD模型小鼠中的AMPAR内吞作用有效地改善了认知和突触功能,并且可能是用于AD治疗的新分子。辣椒素(1 mg / kg,腹膜内)(TRPV1的激动剂)对TRPV1的药理激活极大地逆转了AD模型小鼠的海马CA1 LTP损伤和空间学习与记忆。综上所述,这些结果表明TRPV1激活通过抑制AD模型小鼠中的AMPAR内吞作用有效地改善了认知和突触功能,并且可能是用于AD治疗的新分子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号