当前位置:
X-MOL 学术
›
Magn. Reson. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Qualitative and Quantitative 1 H NMR Spectroscopy for Determination of Divalent Metal Cation Concentration in Model Salt Solutions, Food Supplements and Pharmaceutical Products by Using EDTA as Chelating Agent
Magnetic Resonance in Chemistry ( IF 1.9 ) Pub Date : 2020-03-11 , DOI: 10.1002/mrc.5009 Elina Hafer 1, 2 , Ulrike Holzgrabe 2 , Katharina Kraus 1 , Kristie Adams 3 , James M Hook 4 , Bernd Diehl 1
Magnetic Resonance in Chemistry ( IF 1.9 ) Pub Date : 2020-03-11 , DOI: 10.1002/mrc.5009 Elina Hafer 1, 2 , Ulrike Holzgrabe 2 , Katharina Kraus 1 , Kristie Adams 3 , James M Hook 4 , Bernd Diehl 1
Affiliation
|
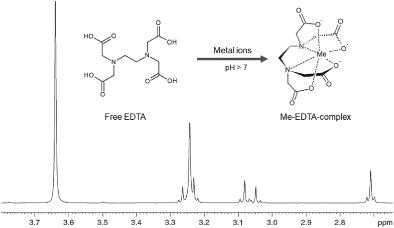
This paper introduces an 1H NMR method to identify individual divalent metal cations Be2+, Mg2+, Ca2+, Sr2+, Zn2+, Cd2+, Hg2+, Sn2+, and Pb2+ in aqueous salt solutions through their unique signal shift and coupling after complexation with the salt of ethylenediaminetetraacetic acid (EDTA). Furthermore, quantitative determination applied for the divalent metal cations Ca2+, Mg2+, Hg2+, Sn2+, Pb2+, and Zn2+ (limit of quantification: 5–22 μg/ml) can be achieved using an excess of EDTA with aqueous model salt solutions. An internal standard is not required because a known excess of EDTA is added and the remaining free EDTA can be used to recalculate the quantity of chelated metal cations. The utility of the method is demonstrated for the analysis of divalent cations in some food supplements and in pharmaceutical products.
中文翻译:

使用 EDTA 作为螯合剂,通过定性和定量 1 H NMR 光谱测定模型盐溶液、食品补充剂和药品中的二价金属阳离子浓度
本文介绍了一种 1H NMR 方法,通过与乙二胺四乙酸盐络合后的独特信号位移和偶联,识别盐水溶液中的单个二价金属阳离子 Be2+、Mg2+、Ca2+、Sr2+、Zn2+、Cd2+、Hg2+、Sn2+ 和 Pb2+ (乙二胺四乙酸)。此外,可以使用过量的 EDTA 和模型盐水溶液对二价金属阳离子 Ca2+、Mg2+、Hg2+、Sn2+、Pb2+ 和 Zn2+(定量限:5–22 μg/ml)进行定量测定。不需要内标,因为添加了已知过量的 EDTA,剩余的游离 EDTA 可用于重新计算螯合金属阳离子的数量。该方法可用于分析某些食品补充剂和药品中的二价阳离子。
更新日期:2020-03-11
中文翻译:

使用 EDTA 作为螯合剂,通过定性和定量 1 H NMR 光谱测定模型盐溶液、食品补充剂和药品中的二价金属阳离子浓度
本文介绍了一种 1H NMR 方法,通过与乙二胺四乙酸盐络合后的独特信号位移和偶联,识别盐水溶液中的单个二价金属阳离子 Be2+、Mg2+、Ca2+、Sr2+、Zn2+、Cd2+、Hg2+、Sn2+ 和 Pb2+ (乙二胺四乙酸)。此外,可以使用过量的 EDTA 和模型盐水溶液对二价金属阳离子 Ca2+、Mg2+、Hg2+、Sn2+、Pb2+ 和 Zn2+(定量限:5–22 μg/ml)进行定量测定。不需要内标,因为添加了已知过量的 EDTA,剩余的游离 EDTA 可用于重新计算螯合金属阳离子的数量。该方法可用于分析某些食品补充剂和药品中的二价阳离子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号