当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction of triols with formaldehyde and acetone: Experimental and theoretical study
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2020-02-16 , DOI: 10.1002/jccs.201900401 Rimma Sultanova 1 , Sophia Borisevich 1 , Gulnara Raskil'dina 2 , Julianna Borisova 2 , Irina Baykova 1 , Leonid Spirikhin 1 , Sergey Khursan 1 , Simon Zlotsky 2
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2020-02-16 , DOI: 10.1002/jccs.201900401 Rimma Sultanova 1 , Sophia Borisevich 1 , Gulnara Raskil'dina 2 , Julianna Borisova 2 , Irina Baykova 1 , Leonid Spirikhin 1 , Sergey Khursan 1 , Simon Zlotsky 2
Affiliation
|
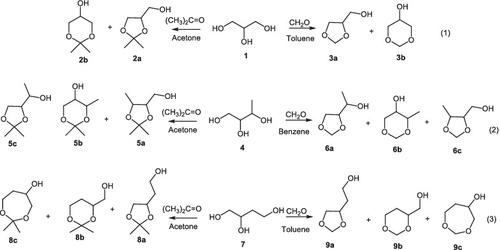
Experimental and theoretical aspects of the condensation of glycerol and its homologs (1,2,3‐ and 1,2,4‐butanetriols) with formaldehyde and acetone are studied under conditions of acid catalysis. Calculation of the thermodynamic parameters of the resulting products by the composite method CBS‐QB3 shows that the six‐membered heterocycles, the products of the interaction of triols with formaldehyde, are thermodynamically more stable than the five‐membered acetals, while the reaction of the same triols with acetone is preferable for the formation of the five‐membered acetals. This is due to the fact that the regioselectivity of the studied reactions is determined by the structural features and reactivity of the carbocations formed in a condensed medium during the course of the reaction. According to the theoretical data obtained experimentally, during the condensation of glycerol and 1,2,4‐butanetriol with formaldehyde in the most stable form of the six‐membered cyclic carbocation, intramolecular hydrogen bonding and anomeric stabilization due to the axially oriented hydroxyl group take place. As a result, cation 1b–1 is 1.2–1.6 kJ/mol more stable than its five‐membered isomers (1a–1 and 1b–2 ). It leads to the predominant formation of 1,3‐dioxane (3b ). However, upon condensation of butanetriol‐1,2,3 with formaldehyde, the intermediate cation 4a–1 turns out to be significantly more stable than the other isomers due to the strong intramolecular hydrogen bond in the six‐membered ring with the participation of the hydroxyl group of the substituent and the hydroxyl group of the cationic center, leading to the predominant formation of the dioxolane 6a .
中文翻译:

三醇与甲醛和丙酮的相互作用:实验和理论研究
在酸催化条件下,研究了甘油及其同系物(1,2,3-和1,2,4-丁三醇)与甲醛和丙酮缩合的实验和理论方面。用复合方法CBS-QB3计算所得产物的热力学参数表明,三元醇与甲醛相互作用的产物六元杂环比五元乙缩醛在热力学上更稳定,而相同的三元醇与丙酮更适合形成五元缩醛。这是由于以下事实:所研究的反应的区域选择性取决于反应过程中在冷凝介质中形成的碳阳离子的结构特征和反应性。根据实验获得的理论数据,在甘油和1,2,4-丁三醇与甲醛以最稳定的六元环状碳正离子形式缩合的过程中,由于轴向取向的羟基,分子内氢键和异头稳定化发生。结果,阳离子1b-1比其五元异构体(1a-1和1b-2)稳定1.2-1.6 kJ / mol 。它导致1,3-二恶烷(3b)的主要形成。但是,丁三醇-1,2,3与甲醛缩合后,由于六元环中强大的分子内氢键以及氢键的参与,中间阳离子4a-1明显比其他异构体更稳定。取代基的羟基和阳离子中心的羟基,导致二氧戊环6a的主要形成。
更新日期:2020-02-16
中文翻译:

三醇与甲醛和丙酮的相互作用:实验和理论研究
在酸催化条件下,研究了甘油及其同系物(1,2,3-和1,2,4-丁三醇)与甲醛和丙酮缩合的实验和理论方面。用复合方法CBS-QB3计算所得产物的热力学参数表明,三元醇与甲醛相互作用的产物六元杂环比五元乙缩醛在热力学上更稳定,而相同的三元醇与丙酮更适合形成五元缩醛。这是由于以下事实:所研究的反应的区域选择性取决于反应过程中在冷凝介质中形成的碳阳离子的结构特征和反应性。根据实验获得的理论数据,在甘油和1,2,4-丁三醇与甲醛以最稳定的六元环状碳正离子形式缩合的过程中,由于轴向取向的羟基,分子内氢键和异头稳定化发生。结果,阳离子1b-1比其五元异构体(1a-1和1b-2)稳定1.2-1.6 kJ / mol 。它导致1,3-二恶烷(3b)的主要形成。但是,丁三醇-1,2,3与甲醛缩合后,由于六元环中强大的分子内氢键以及氢键的参与,中间阳离子4a-1明显比其他异构体更稳定。取代基的羟基和阳离子中心的羟基,导致二氧戊环6a的主要形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号