当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, spectroscopic characterization, and in vitro antimicrobial activity of fused pyrazolo[4′,3′:4,5]thieno[3,2‐d]pyrimidine
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2020-02-16 , DOI: 10.1002/jccs.201900374 Ahmed F. Saber 1 , Adel M. Kamal El‐Dean 1 , Shaban M. Redwan 1 , Remon M. Zaki 1
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2020-02-16 , DOI: 10.1002/jccs.201900374 Ahmed F. Saber 1 , Adel M. Kamal El‐Dean 1 , Shaban M. Redwan 1 , Remon M. Zaki 1
Affiliation
|
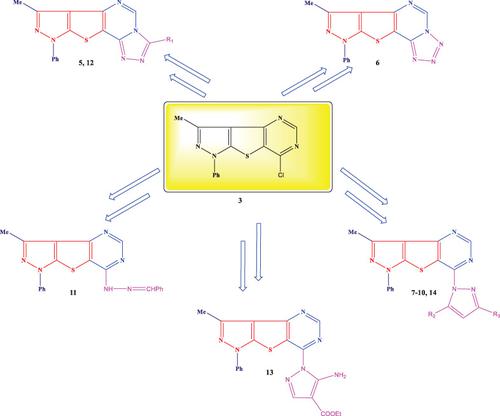
4‐Amino‐3‐methyl‐1‐phenyl‐1H ‐thieno[2,3‐c ]pyrazole‐5‐carboxamide (1 ), which had been previously synthesized according to literature, was used for synthesizing pyrazolothieno‐pyrimidine (2 ) in the presence of triethyl orthoformate and acetic acid. Chlorination of the latter compound upon reflux with phosphorus oxychloride afforded the chloropyrazolothienopyrimidine (3 ), which underwent heterocyclization reaction with sodium azide to produce the tetrazolo‐pyrazolothienopyrimidine (6 ). The chloropyrimidine (3 ) reacted with hydrazine hydrate to give the hydrazinopyrimidine derivative (4 ), which in turn underwent intramolecular condensation reactions with various 1,3‐dicarbonyl compounds, namely ethyl acetoacetate, ethyl benzoylacetate, ethyl cyanoacetate, acetylacetone, diethyl malonate, and ethyl (ethoxymethylene) cyanoacetate, yielding new pyrazolyl pyrazolothienopyrimidine ring systems. Also triazolopyrazolothieno‐pyrimidines and benzylidene Schiff's base compounds were obtained as a result of the reactions with carbon disulfide in pyridine and benzaldehyde, respectively. The chemical structures of the newly synthesized compounds were elucidated using elemental and spectroscopic analyses (FT‐IR, 1 H‐NMR, 13 C‐NMR, and mass spectroscopy). Some of the synthesized compounds possess high antibacterial and antifungal activities.
中文翻译:

稠合吡唑并[4',3':4,5]噻吩并[3,2-d]嘧啶的合成,光谱表征和体外抗菌活性
根据文献先前合成的4-氨基3-甲基-1-苯基-1 H-噻吩并[2,3 - c ]吡唑-5-羧酰胺(1)用于合成吡唑并噻吩并嘧啶(2)在原甲酸三乙酯和乙酸的存在下。后一种化合物在与三氯氧化磷回流后进行氯化,得到氯吡唑并噻吩并嘧啶(3),后者与叠氮化钠进行杂环反应以生成四唑并吡唑并噻吩并并嘧啶(6)。氯嘧啶(3)与水合肼反应生成肼基嘧啶衍生物(4),然后与各种1,3-二羰基化合物进行分子内缩合反应,即乙酰乙酸乙酯,苯甲酰基乙酸乙酯,氰基乙酸乙酯,乙酰丙酮,二乙基丙二酸酯和(乙氧基亚甲基)氰乙酸乙酯,生成新的吡唑基吡唑并噻吩并嘧啶环体系。通过与二硫化碳分别在吡啶和苯甲醛中的反应,还获得了三唑并吡唑并硫嘧啶和亚苄基席夫碱化合物。使用元素分析和光谱分析(FT-IR,1 H-NMR,13 C-NMR和质谱)阐明了新合成化合物的化学结构。一些合成的化合物具有很高的抗菌和抗真菌活性。
更新日期:2020-02-16
中文翻译:

稠合吡唑并[4',3':4,5]噻吩并[3,2-d]嘧啶的合成,光谱表征和体外抗菌活性
根据文献先前合成的4-氨基3-甲基-1-苯基-1 H-噻吩并[2,3 - c ]吡唑-5-羧酰胺(1)用于合成吡唑并噻吩并嘧啶(2)在原甲酸三乙酯和乙酸的存在下。后一种化合物在与三氯氧化磷回流后进行氯化,得到氯吡唑并噻吩并嘧啶(3),后者与叠氮化钠进行杂环反应以生成四唑并吡唑并噻吩并并嘧啶(6)。氯嘧啶(3)与水合肼反应生成肼基嘧啶衍生物(4),然后与各种1,3-二羰基化合物进行分子内缩合反应,即乙酰乙酸乙酯,苯甲酰基乙酸乙酯,氰基乙酸乙酯,乙酰丙酮,二乙基丙二酸酯和(乙氧基亚甲基)氰乙酸乙酯,生成新的吡唑基吡唑并噻吩并嘧啶环体系。通过与二硫化碳分别在吡啶和苯甲醛中的反应,还获得了三唑并吡唑并硫嘧啶和亚苄基席夫碱化合物。使用元素分析和光谱分析(FT-IR,1 H-NMR,13 C-NMR和质谱)阐明了新合成化合物的化学结构。一些合成的化合物具有很高的抗菌和抗真菌活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号