当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Towards Sarpagine-Ajmaline-Macroline Family Indole Alkaloids: Enantioselective Synthesis of N-Demethyl Alstolactone Diastereomer.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-03-25 , DOI: 10.1002/chem.202000415 Dylan Dagoneau 1 , Qian Wang 1 , Jieping Zhu 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-03-25 , DOI: 10.1002/chem.202000415 Dylan Dagoneau 1 , Qian Wang 1 , Jieping Zhu 1
Affiliation
|
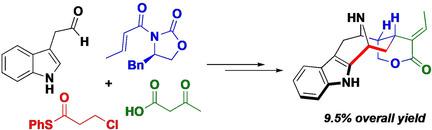
We report herein our strategy aiming at using the functionalized tetrahydro-6H-cycloocta[b]indol-6-one as a key intermediate for the synthesis of sarpagine-ajmaline-macroline family of monoterpene indole alkaloids. The desired tricycle was synthesized via following key steps: a) Evans' syn-selective aldolization; b) Liebeskind-Srogl cross-coupling using phenylthiol ester of 3-chloropropanoic acid as a surrogate of acrylic thioester for the synthesis of 2,3-disubstituted indoles; c) ring-closing metathesis (RCM) for the formation of the 8-membered ring. An N-allylation followed by intramolecular 1,4-addition was planned for the synthesis of vobasine class of natural products. However, cyclization under a diverse set of conditions involving anionic, radical and organopalladium/organonickel species failed to produce the bridged ring system. On the other hand, esterification of the pendent primary alcohol with acetoacetic acid followed by intramolecular Michael addition afforded the desired tetracycle with an excellent diastereoselectivity. Subsequent functional group manipulation and transannular cyclization of the amino alcohol afforded the N(1)-demethyl-3,5-diepi-alstolactone. We believe that the same synthetic route would afford the alstolactone should the amino alcohol with appropriate stereochemistry be used as a starting material.
中文翻译:

走向Sarpagine-Ajmaline-Macroline家族吲哚生物碱:N-脱甲基Altolactone非对映异构体的对映选择性合成。
我们在这里报告了我们的策略,旨在使用功能化的四氢-6H-环辛基[b]吲哚-6-作为合成萜烯吲哚-大麦碱单萜吲哚生物碱的关键中间体。通过以下关键步骤合成了所需的三轮车:a)Evans的顺选择性醛醇缩合;b)使用3-氯丙酸的苯硫酚酯作为丙烯酸硫酯的代用品来合成2,3-二取代的吲哚,进行Liebeskind-Srogl交叉偶联。c)用于形成8元环的闭环易位(RCM)。计划将N-烯丙基化,然后进行分子内1,4-添加,以合成vobasine类天然产物。然而,在涉及阴离子,自由基和有机钯/有机基酮种类的多种条件下的环化未能产生桥环系统。另一方面,将侧基伯醇与乙酰乙酸酯化,然后分子内迈克尔加成,得到所需的四环具有非对映选择性。随后的官能团操纵和氨基醇的环过环化提供了N(1)-demethyl-3,5-diepi-alstolactone。我们认为,如果具有适当立体化学的氨基醇被用作起始原料,则相同的合成途径将提供戊内酯。
更新日期:2020-03-27
中文翻译:

走向Sarpagine-Ajmaline-Macroline家族吲哚生物碱:N-脱甲基Altolactone非对映异构体的对映选择性合成。
我们在这里报告了我们的策略,旨在使用功能化的四氢-6H-环辛基[b]吲哚-6-作为合成萜烯吲哚-大麦碱单萜吲哚生物碱的关键中间体。通过以下关键步骤合成了所需的三轮车:a)Evans的顺选择性醛醇缩合;b)使用3-氯丙酸的苯硫酚酯作为丙烯酸硫酯的代用品来合成2,3-二取代的吲哚,进行Liebeskind-Srogl交叉偶联。c)用于形成8元环的闭环易位(RCM)。计划将N-烯丙基化,然后进行分子内1,4-添加,以合成vobasine类天然产物。然而,在涉及阴离子,自由基和有机钯/有机基酮种类的多种条件下的环化未能产生桥环系统。另一方面,将侧基伯醇与乙酰乙酸酯化,然后分子内迈克尔加成,得到所需的四环具有非对映选择性。随后的官能团操纵和氨基醇的环过环化提供了N(1)-demethyl-3,5-diepi-alstolactone。我们认为,如果具有适当立体化学的氨基醇被用作起始原料,则相同的合成途径将提供戊内酯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号