当前位置:
X-MOL 学术
›
Nat. Struct. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The structure of the cohesin ATPase elucidates the mechanism of SMC-kleisin ring opening.
Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2020-02-17 , DOI: 10.1038/s41594-020-0379-7 Kyle W Muir 1, 2 , Yan Li 1 , Felix Weis 3 , Daniel Panne 1, 4
Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2020-02-17 , DOI: 10.1038/s41594-020-0379-7 Kyle W Muir 1, 2 , Yan Li 1 , Felix Weis 3 , Daniel Panne 1, 4
Affiliation
|
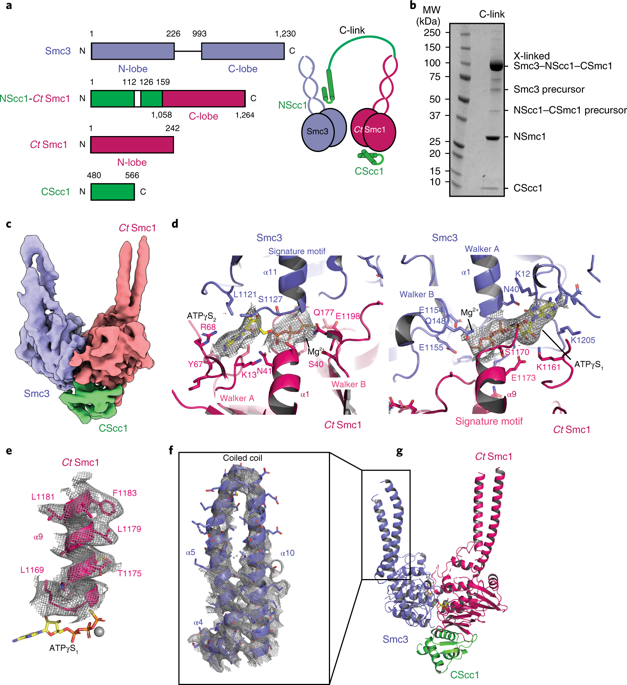
Genome regulation requires control of chromosome organization by SMC-kleisin complexes. The cohesin complex contains the Smc1 and Smc3 subunits that associate with the kleisin Scc1 to form a ring-shaped complex that can topologically engage chromatin to regulate chromatin structure. Release from chromatin involves opening of the ring at the Smc3-Scc1 interface in a reaction that is controlled by acetylation and engagement of the Smc ATPase head domains. To understand the underlying molecular mechanisms, we have determined the 3.2-Å resolution cryo-electron microscopy structure of the ATPγS-bound, heterotrimeric cohesin ATPase head module and the 2.1-Å resolution crystal structure of a nucleotide-free Smc1-Scc1 subcomplex from Saccharomyces cerevisiae and Chaetomium thermophilium. We found that ATP-binding and Smc1-Smc3 heterodimerization promote conformational changes within the ATPase that are transmitted to the Smc coiled-coil domains. Remodeling of the coiled-coil domain of Smc3 abrogates the binding surface for Scc1, thus leading to ring opening at the Smc3-Scc1 interface.
中文翻译:

粘连蛋白 ATP 酶的结构阐明了 SMC-kleisin 开环的机制。
基因组调控需要 SMC-kleisin 复合物控制染色体组织。粘连蛋白复合物包含 Smc1 和 Smc3 亚基,它们与 kleisin Scc1 结合形成环形复合物,可以拓扑地接合染色质以调节染色质结构。从染色质释放涉及在 Smc3-Scc1 界面处打开环的反应,该反应由 Smc ATPase 头域的乙酰化和接合控制。为了了解潜在的分子机制,我们确定了 ATPγS 结合的异三聚粘连蛋白 ATPase 头模块的 3.2 Å 分辨率冷冻电子显微镜结构,以及来自酵母菌的无核苷酸 Smc1-Scc1 亚复合物的 2.1 Å 分辨率晶体结构酿酒酵母和嗜热毛壳菌。我们发现 ATP 结合和 Smc1-Smc3 异二聚化促进 ATP 酶内的构象变化,并传递到 Smc 卷曲螺旋结构域。 Smc3 卷曲螺旋结构域的重塑消除了 Scc1 的结合表面,从而导致 Smc3-Scc1 界面处开环。
更新日期:2020-02-17
中文翻译:

粘连蛋白 ATP 酶的结构阐明了 SMC-kleisin 开环的机制。
基因组调控需要 SMC-kleisin 复合物控制染色体组织。粘连蛋白复合物包含 Smc1 和 Smc3 亚基,它们与 kleisin Scc1 结合形成环形复合物,可以拓扑地接合染色质以调节染色质结构。从染色质释放涉及在 Smc3-Scc1 界面处打开环的反应,该反应由 Smc ATPase 头域的乙酰化和接合控制。为了了解潜在的分子机制,我们确定了 ATPγS 结合的异三聚粘连蛋白 ATPase 头模块的 3.2 Å 分辨率冷冻电子显微镜结构,以及来自酵母菌的无核苷酸 Smc1-Scc1 亚复合物的 2.1 Å 分辨率晶体结构酿酒酵母和嗜热毛壳菌。我们发现 ATP 结合和 Smc1-Smc3 异二聚化促进 ATP 酶内的构象变化,并传递到 Smc 卷曲螺旋结构域。 Smc3 卷曲螺旋结构域的重塑消除了 Scc1 的结合表面,从而导致 Smc3-Scc1 界面处开环。











































 京公网安备 11010802027423号
京公网安备 11010802027423号