当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Capture and Reactivity of an Elusive Carbon-Sulfur Centered Biradical.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-02-17 , DOI: 10.1021/acs.jpca.9b11795 Dennis Gerbig 1 , Bastian Bernhardt 1 , Raffael C Wende 1 , Peter R Schreiner 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-02-17 , DOI: 10.1021/acs.jpca.9b11795 Dennis Gerbig 1 , Bastian Bernhardt 1 , Raffael C Wende 1 , Peter R Schreiner 1
Affiliation
|
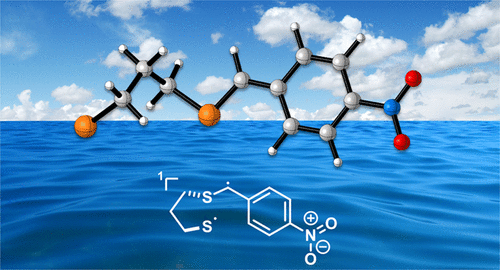
The initial oxidation product of dimethyl sulfide in the marine boundary layer, the methyl thiomethyl radical, has remained elusive. A structurally analogous biradical with one radical center in the α-position to a sulfur atom could now be obtained by UV irradiation of p-nitrobenzaldehyde dithiane isolated in solid dinitrogen (N2) or Ar at cryogenic temperatures. A spin-forbidden reaction with triplet dioxygen (3O2) does not occur. The dithiane of o-nitrobenzaldehyde rather undergoes a series of rearrangements under the same conditions, resulting in overall photodeprotection.
中文翻译:

难以捉摸的以碳硫为中心的双自由基的捕获和反应性。
海洋边界层中的二甲基硫醚的初始氧化产物甲基硫代甲基自由基仍然难以捉摸。现在,可以通过在低温下在固态二氮(N2)或Ar中分离的对硝基苯甲醛二噻吩的紫外线辐射,获得在硫原子的α位上一个自由基中心位于硫原子上的结构类似的双自由基。不会发生与三重态双氧(3O2)的自旋禁止反应。邻硝基苯甲醛的二噻吩在相同条件下反而经历了一系列重排,从而导致整体光脱保护。
更新日期:2020-03-03
中文翻译:

难以捉摸的以碳硫为中心的双自由基的捕获和反应性。
海洋边界层中的二甲基硫醚的初始氧化产物甲基硫代甲基自由基仍然难以捉摸。现在,可以通过在低温下在固态二氮(N2)或Ar中分离的对硝基苯甲醛二噻吩的紫外线辐射,获得在硫原子的α位上一个自由基中心位于硫原子上的结构类似的双自由基。不会发生与三重态双氧(3O2)的自旋禁止反应。邻硝基苯甲醛的二噻吩在相同条件下反而经历了一系列重排,从而导致整体光脱保护。











































 京公网安备 11010802027423号
京公网安备 11010802027423号