当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biophysical Techniques for Distinguishing Ligand Binding Modes in Cytochrome P450 Monooxygenases.
Biochemistry ( IF 2.9 ) Pub Date : 2020-02-24 , DOI: 10.1021/acs.biochem.0c00027 Matthew N Podgorski 1 , Joshua S Harbort 2 , Tom Coleman 1 , Jeanette E Stok 3 , Jake A Yorke 4 , Luet-Lok Wong 4 , John B Bruning 5 , Paul V Bernhardt 3 , James J De Voss 3 , Jeffrey R Harmer 2 , Stephen G Bell 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-02-24 , DOI: 10.1021/acs.biochem.0c00027 Matthew N Podgorski 1 , Joshua S Harbort 2 , Tom Coleman 1 , Jeanette E Stok 3 , Jake A Yorke 4 , Luet-Lok Wong 4 , John B Bruning 5 , Paul V Bernhardt 3 , James J De Voss 3 , Jeffrey R Harmer 2 , Stephen G Bell 1
Affiliation
|
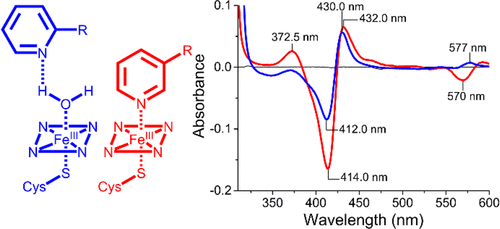
The cytochrome P450 superfamily of heme monooxygenases catalyzes important chemical reactions across nature. The changes in the optical spectra of these enzymes, induced by the addition of substrates or inhibitors, are critical for assessing how these molecules bind to the P450, enhancing or inhibiting the catalytic cycle. Here we use the bacterial CYP199A4 enzyme (Uniprot entry Q2IUO2 ), from Rhodopseudomonas palustris HaA2, and a range of substituted benzoic acids to investigate different binding modes. 4-Methoxybenzoic acid elicits an archetypal type I spectral response due to a ≥95% switch from the low- to high-spin state with concomitant dissociation of the sixth aqua ligand. 4-(Pyridin-3-yl)- and 4-(pyridin-2-yl)benzoic acid induced different type II ultraviolet-visible (UV-vis) spectral responses in CYP199A4. The former induced a greater red shift in the Soret wavelength (424 nm vs 422 nm) along with a larger overall absorbance change and other differences in the α-, β-, and δ-bands. There were also variations in the ferrous UV-vis spectra of these two substrate-bound forms with a spectrum indicative of Fe-N bond formation with 4-(pyridin-3-yl)benzoic acid. The crystal structures of CYP199A4, with the pyridinyl compounds bound, revealed that while the nitrogen of 4-(pyridin-3-yl)benzoic acid is coordinated to the heme, with 4-(pyridin-2-yl)benzoic acid an aqua ligand remains. Continuous wave and pulse electron paramagnetic resonance data in frozen solution revealed that the substrates are bound in the active site in a form consistent with the crystal structures. The redox potential of each CYP199A4-substrate combination was measured, allowing correlation among binding modes, spectroscopic properties, and the observed biochemical activity.
中文翻译:

区分细胞色素P450单加氧酶中配体结合模式的生物物理技术。
血红素单加氧酶的细胞色素P450超家族催化整个自然界的重要化学反应。这些酶的光谱变化是由底物或抑制剂的添加引起的,对于评估这些分子如何与P450结合,增强或抑制催化循环至关重要。在这里,我们使用细菌CYP199A4酶(Uniprot条目Q2IUO2)(来自Rhodopseudomonas palustris HaA2)和一系列取代的苯甲酸来研究不同的结合模式。4-甲氧基苯甲酸可引起原型I型光谱响应,这是由于从低旋态到高旋态的转换率≥95%,同时伴随着第六水基配体的解离。4-(吡啶-3-基)-和4-(吡啶-2-基)苯甲酸在CYP199A4中引起不同的II型紫外可见(UV-vis)光谱响应。前者在Soret波长(424 nm对422 nm)中引起较大的红移,同时在α波段,β波段和δ波段中出现了更大的总体吸光度变化和其他差异。这两种底物结合形式的亚铁紫外-可见光谱也有变化,其光谱表明与4-(吡啶-3-基)苯甲酸形成了Fe-N键。结合有吡啶基化合物的CYP199A4的晶体结构表明,虽然4-(吡啶-3-基)苯甲酸的氮与血红素配位,而4-(吡啶-2-基)苯甲酸是一种水配体遗迹。冷冻溶液中的连续波和脉冲电子顺磁共振数据表明,基质以与晶体结构一致的形式结合在活性位点中。测量每种CYP199A4底物组合的氧化还原电势,
更新日期:2020-02-24
中文翻译:

区分细胞色素P450单加氧酶中配体结合模式的生物物理技术。
血红素单加氧酶的细胞色素P450超家族催化整个自然界的重要化学反应。这些酶的光谱变化是由底物或抑制剂的添加引起的,对于评估这些分子如何与P450结合,增强或抑制催化循环至关重要。在这里,我们使用细菌CYP199A4酶(Uniprot条目Q2IUO2)(来自Rhodopseudomonas palustris HaA2)和一系列取代的苯甲酸来研究不同的结合模式。4-甲氧基苯甲酸可引起原型I型光谱响应,这是由于从低旋态到高旋态的转换率≥95%,同时伴随着第六水基配体的解离。4-(吡啶-3-基)-和4-(吡啶-2-基)苯甲酸在CYP199A4中引起不同的II型紫外可见(UV-vis)光谱响应。前者在Soret波长(424 nm对422 nm)中引起较大的红移,同时在α波段,β波段和δ波段中出现了更大的总体吸光度变化和其他差异。这两种底物结合形式的亚铁紫外-可见光谱也有变化,其光谱表明与4-(吡啶-3-基)苯甲酸形成了Fe-N键。结合有吡啶基化合物的CYP199A4的晶体结构表明,虽然4-(吡啶-3-基)苯甲酸的氮与血红素配位,而4-(吡啶-2-基)苯甲酸是一种水配体遗迹。冷冻溶液中的连续波和脉冲电子顺磁共振数据表明,基质以与晶体结构一致的形式结合在活性位点中。测量每种CYP199A4底物组合的氧化还原电势,











































 京公网安备 11010802027423号
京公网安备 11010802027423号