当前位置:
X-MOL 学术
›
Pharmacogenomics J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combining clinical and candidate gene data into a risk score for azathioprine-associated leukopenia in routine clinical practice.
The Pharmacogenomics Journal ( IF 2.9 ) Pub Date : 2020-02-14 , DOI: 10.1038/s41397-020-0163-4 Prathima Anandi 1 , Alyson L Dickson 1 , QiPing Feng 1 , Wei-Qi Wei 2 , William D Dupont 3 , Dale Plummer 3 , Ge Liu 1 , Rany Octaria 1 , Katherine A Barker 1 , Vivian K Kawai 1 , Kelly Birdwell 1 , Nancy J Cox 1 , Adriana Hung 1 , C Michael Stein 1 , Cecilia P Chung 1
The Pharmacogenomics Journal ( IF 2.9 ) Pub Date : 2020-02-14 , DOI: 10.1038/s41397-020-0163-4 Prathima Anandi 1 , Alyson L Dickson 1 , QiPing Feng 1 , Wei-Qi Wei 2 , William D Dupont 3 , Dale Plummer 3 , Ge Liu 1 , Rany Octaria 1 , Katherine A Barker 1 , Vivian K Kawai 1 , Kelly Birdwell 1 , Nancy J Cox 1 , Adriana Hung 1 , C Michael Stein 1 , Cecilia P Chung 1
Affiliation
|
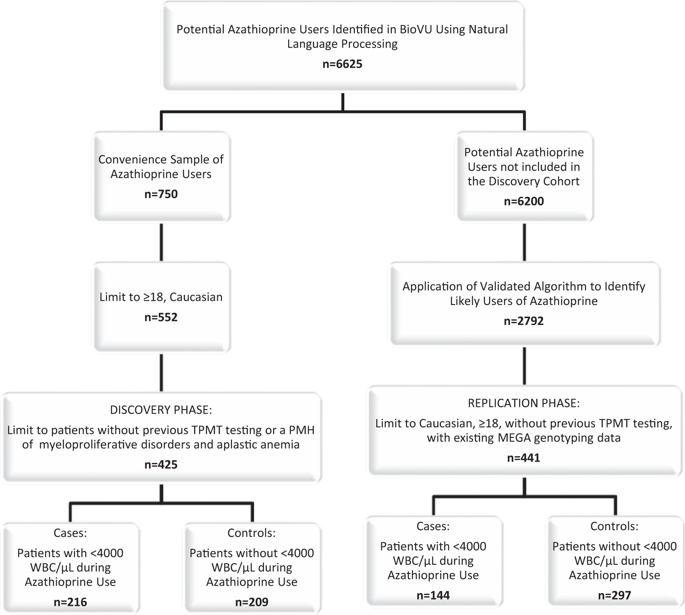
Leukopenia is a serious, frequent side effect associated with azathioprine use. Currently, we use thiopurine methyltransferase (TPMT) testing to predict leukopenia in patients taking azathioprine. We hypothesized that a risk score incorporating additional clinical and genetic variables would improve the prediction of azathioprine-associated leukopenia. In the discovery phase, we developed four risk score models: (1) age, sex, and TPMT metabolizer status; (2) model 1 plus additional clinical variables; (3) sixty candidate single nucleotide polymorphisms; and (4) model 2 plus model 3. The area under the receiver-operating-characteristic curve (AUC) of the risk scores was 0.59 (95% CI: 0.54-0.64), 0.75 (0.71-0.80), 0.66 (0.61-0.71), and 0.78 (0.74-0.82) for models 1, 2, 3, and 4, respectively. During the replication phase, models 2 and 4 (AUC = 0.64, 95% CI: 0.59-0.70 and AUC = 0.63, 95% CI: 0.58-0.69, respectively) were significant in an independent group. Compared with TPMT testing alone, additional genetic and clinical variables improve the prediction of azathioprine-associated leukopenia.
中文翻译:

在常规临床实践中将临床和候选基因数据组合成硫唑嘌呤相关白细胞减少症的风险评分。
白细胞减少症是一种与使用硫唑嘌呤相关的严重且常见的副作用。目前,我们使用硫嘌呤甲基转移酶 (TPMT) 检测来预测服用硫唑嘌呤的患者的白细胞减少。我们假设纳入额外临床和遗传变量的风险评分将改善对硫唑嘌呤相关白细胞减少症的预测。在发现阶段,我们开发了四个风险评分模型:(1)年龄、性别和 TPMT 代谢状态;(2) 模型 1 加上额外的临床变量;(3) 六十个候选单核苷酸多态性;(4) 模型 2 加模型 3。风险评分的受试者工作特征曲线下面积 (AUC) 分别为 0.59 (95% CI: 0.54-0.64)、0.75 (0.71-0.80)、0.66 (0.61- 0.71) 和 0.78 (0.74-0.82) 分别适用于模型 1、2、3 和 4。在复制阶段,模型 2 和 4(AUC = 0.64,95% CI:0.59-0.70 和 AUC = 0.63,95% CI:0.58-0.69,分别)在独立组中显着。与单独的 TPMT 检测相比,额外的遗传和临床变量可提高对硫唑嘌呤相关白细胞减少症的预测。
更新日期:2020-02-14
中文翻译:

在常规临床实践中将临床和候选基因数据组合成硫唑嘌呤相关白细胞减少症的风险评分。
白细胞减少症是一种与使用硫唑嘌呤相关的严重且常见的副作用。目前,我们使用硫嘌呤甲基转移酶 (TPMT) 检测来预测服用硫唑嘌呤的患者的白细胞减少。我们假设纳入额外临床和遗传变量的风险评分将改善对硫唑嘌呤相关白细胞减少症的预测。在发现阶段,我们开发了四个风险评分模型:(1)年龄、性别和 TPMT 代谢状态;(2) 模型 1 加上额外的临床变量;(3) 六十个候选单核苷酸多态性;(4) 模型 2 加模型 3。风险评分的受试者工作特征曲线下面积 (AUC) 分别为 0.59 (95% CI: 0.54-0.64)、0.75 (0.71-0.80)、0.66 (0.61- 0.71) 和 0.78 (0.74-0.82) 分别适用于模型 1、2、3 和 4。在复制阶段,模型 2 和 4(AUC = 0.64,95% CI:0.59-0.70 和 AUC = 0.63,95% CI:0.58-0.69,分别)在独立组中显着。与单独的 TPMT 检测相比,额外的遗传和临床变量可提高对硫唑嘌呤相关白细胞减少症的预测。











































 京公网安备 11010802027423号
京公网安备 11010802027423号