当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrolyte Lifetime in Aqueous Organic Redox Flow Batteries: A Critical Review.
Chemical Reviews ( IF 51.4 ) Pub Date : 2020-02-13 , DOI: 10.1021/acs.chemrev.9b00599 David G Kwabi 1 , Yunlong Ji 2 , Michael J Aziz 3
Chemical Reviews ( IF 51.4 ) Pub Date : 2020-02-13 , DOI: 10.1021/acs.chemrev.9b00599 David G Kwabi 1 , Yunlong Ji 2 , Michael J Aziz 3
Affiliation
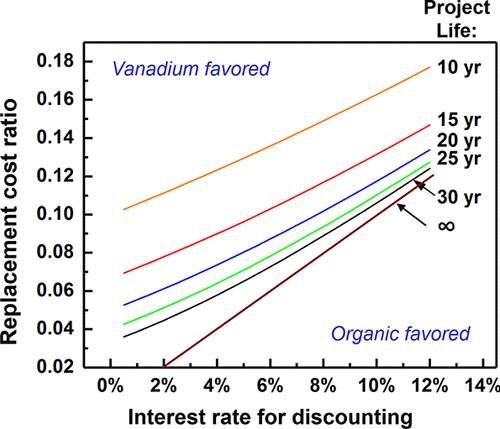
|
Aqueous organic redox flow batteries (RFBs) could enable widespread integration of renewable energy, but only if costs are sufficiently low. Because the levelized cost of storage for an RFB is a function of electrolyte lifetime, understanding and improving the chemical stability of active reactants in RFBs is a critical research challenge. We review known or hypothesized molecular decomposition mechanisms for all five classes of aqueous redox-active organics and organometallics for which cycling lifetime results have been reported: quinones, viologens, aza-aromatics, iron coordination complexes, and nitroxide radicals. We collect, analyze, and compare capacity fade rates from all aqueous organic electrolytes that have been utilized in the capacity-limiting side of flow or hybrid flow/nonflow cells, noting also their redox potentials and demonstrated concentrations of transferrable electrons. We categorize capacity fade rates as being “high” (>1%/day), “moderate” (0.1–1%/day), “low” (0.02–0.1%/day), and “extremely low” (≤0.02%/day) and discuss the degree to which the fade rates have been linked to decomposition mechanisms. Capacity fade is observed to be time-denominated rather than cycle-denominated, with a temporal rate that can depend on molecular concentrations and electrolyte state of charge through, e.g., bimolecular decomposition mechanisms. We then review measurement methods for capacity fade rate and find that simple galvanostatic charge–discharge cycling is inadequate for assessing capacity fade when fade rates are low or extremely low and recommend refining methods to include potential holds for accurately assessing molecular lifetimes under such circumstances. We consider separately symmetric cell cycling results, the interpretation of which is simplified by the absence of a different counter-electrolyte. We point out the chemistries with low or extremely low established fade rates that also exhibit open circuit potentials of 1.0 V or higher and transferrable electron concentrations of 1.0 M or higher, which are promising performance characteristics for RFB commercialization. We point out important directions for future research.
中文翻译:

水性有机氧化还原液流电池中的电解质寿命:关键评论。
水性有机氧化还原液流电池(RFB)可以实现可再生能源的广泛整合,但前提是成本要足够低。由于RFB的平均存储成本是电解质寿命的函数,因此了解和提高RFB中活性反应物的化学稳定性是一项至关重要的研究挑战。我们审查了已知的或假设的分子分解机理,用于所有五类水性氧化还原活性有机物和有机金属化合物,其循环寿命结果已有报道:醌,紫精,氮杂芳烃,铁配位络合物和一氧化氮自由基。我们收集,分析和比较已在流通池或混合流通/非流通池的容量限制侧使用的所有水性有机电解质的容量衰减率,还注意到它们的氧化还原电势并证明了可转移电子的浓度。我们将容量衰减率分为“高”(> 1%/天),“中”(0.1–1%/天),“低”(0.02–0.1%/天)和“极低”(≤0.02) %/天),并讨论衰落率与分解机制相关的程度。观察到容量衰减是按时间计价的,而不是按周期计价的,其时间速率可能取决于分子浓度和通过例如双分子分解机理的电解质的电荷状态。然后,我们回顾了容量衰减率的测量方法,发现当衰减率较低或极低时,简单的恒电流充放电循环不足以评估容量衰减,因此建议采用精炼方法,以包括在这种情况下可以准确评估分子寿命的潜在保持力。我们分别考虑对称的细胞循环结果,由于缺乏不同的反电解质,简化了解释。我们指出了建立衰落率低或极低的化学物质,它们也表现出1.0 V或更高的开路电势以及1.0 M或更高的可转移电子浓度,这是RFB商业化的有希望的性能特征。我们指出了未来研究的重要方向。我们分别考虑对称的细胞循环结果,由于缺乏不同的反电解质,简化了解释。我们指出了建立衰落率低或极低的化学物质,它们也表现出1.0 V或更高的开路电势以及1.0 M或更高的可转移电子浓度,这是RFB商业化的有希望的性能特征。我们指出了未来研究的重要方向。我们分别考虑对称的细胞循环结果,由于缺乏不同的反电解质,简化了解释。我们指出了建立衰落率低或极低的化学物质,它们也表现出1.0 V或更高的开路电势以及1.0 M或更高的可转移电子浓度,这是RFB商业化的有希望的性能特征。我们指出了未来研究的重要方向。这是RFB商业化的有希望的性能特征。我们指出了未来研究的重要方向。这是RFB商业化的有希望的性能特征。我们指出了未来研究的重要方向。
更新日期:2020-02-13
中文翻译:

水性有机氧化还原液流电池中的电解质寿命:关键评论。
水性有机氧化还原液流电池(RFB)可以实现可再生能源的广泛整合,但前提是成本要足够低。由于RFB的平均存储成本是电解质寿命的函数,因此了解和提高RFB中活性反应物的化学稳定性是一项至关重要的研究挑战。我们审查了已知的或假设的分子分解机理,用于所有五类水性氧化还原活性有机物和有机金属化合物,其循环寿命结果已有报道:醌,紫精,氮杂芳烃,铁配位络合物和一氧化氮自由基。我们收集,分析和比较已在流通池或混合流通/非流通池的容量限制侧使用的所有水性有机电解质的容量衰减率,还注意到它们的氧化还原电势并证明了可转移电子的浓度。我们将容量衰减率分为“高”(> 1%/天),“中”(0.1–1%/天),“低”(0.02–0.1%/天)和“极低”(≤0.02) %/天),并讨论衰落率与分解机制相关的程度。观察到容量衰减是按时间计价的,而不是按周期计价的,其时间速率可能取决于分子浓度和通过例如双分子分解机理的电解质的电荷状态。然后,我们回顾了容量衰减率的测量方法,发现当衰减率较低或极低时,简单的恒电流充放电循环不足以评估容量衰减,因此建议采用精炼方法,以包括在这种情况下可以准确评估分子寿命的潜在保持力。我们分别考虑对称的细胞循环结果,由于缺乏不同的反电解质,简化了解释。我们指出了建立衰落率低或极低的化学物质,它们也表现出1.0 V或更高的开路电势以及1.0 M或更高的可转移电子浓度,这是RFB商业化的有希望的性能特征。我们指出了未来研究的重要方向。我们分别考虑对称的细胞循环结果,由于缺乏不同的反电解质,简化了解释。我们指出了建立衰落率低或极低的化学物质,它们也表现出1.0 V或更高的开路电势以及1.0 M或更高的可转移电子浓度,这是RFB商业化的有希望的性能特征。我们指出了未来研究的重要方向。我们分别考虑对称的细胞循环结果,由于缺乏不同的反电解质,简化了解释。我们指出了建立衰落率低或极低的化学物质,它们也表现出1.0 V或更高的开路电势以及1.0 M或更高的可转移电子浓度,这是RFB商业化的有希望的性能特征。我们指出了未来研究的重要方向。这是RFB商业化的有希望的性能特征。我们指出了未来研究的重要方向。这是RFB商业化的有希望的性能特征。我们指出了未来研究的重要方向。











































 京公网安备 11010802027423号
京公网安备 11010802027423号