当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Human fibroblast-macrophage tissue spheroids demonstrate ratio-dependent fibrotic activity for in vitro fibrogenesis model development.
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-03-31 , DOI: 10.1039/c9bm00900k Yu Tan 1 , Allister Suarez 1 , Matthew Garza 1 , Aadil A Khan 2 , Jennifer Elisseeff 3 , Devin Coon 1
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-03-31 , DOI: 10.1039/c9bm00900k Yu Tan 1 , Allister Suarez 1 , Matthew Garza 1 , Aadil A Khan 2 , Jennifer Elisseeff 3 , Devin Coon 1
Affiliation
|
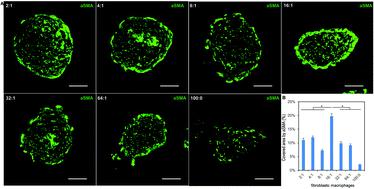
Fibrosis is a pathological accumulation of excessive collagen that underlies many of the most common diseases, representing dysfunction of the essential processes of normal tissue healing. Fibrosis research aims to limit this response without ameliorating the essential role of fibrogenesis in organ function. However, the absence of a realistic in vitro model has hindered investigation into mechanisms and potential interventions because the standard 2D monolayer culture of fibroblasts has limited applicability. We sought to develop and optimize fibrosis spheroids: a scaffold-free three-dimensional human fibroblast-macrophage spheroid system representing an improved benchtop model of human fibrosis. We created, characterized and optimized human fibroblast-only spheroids, demonstrating increased collagen deposition compared to monolayer fibroblasts, while spheroids larger than 300 μm suffered from progressively increasing apoptosis. Next, we improved the spheroid system with the addition of human macrophages to more precisely recapitulate the environment during fibrogenesis, creating a hybrid spheroid system with different ratios of fibroblasts and macrophages ranging from 2 : 1 to 64 : 1. We found that in the hybrid spheroids (particularly the 16 : 1 [F16] ratio) more fibroblasts were activated, with greater macrophage polarization towards a pro-inflammatory M1 phenotype. Hybrid spheroids containing higher ratios of macrophages showed greater macrophage heterogeneity and less fibrogenesis, while low macrophage ratios limited macrophage-induced effects and yielded less collagen deposition. The F16 group also had the highest expression levels of fibrosis-related genes (Col-1a1, Col-3a1 and TGF-β) and inflammation-related genes (TNF, IL1β and IL6). IF staining demonstrated that F16 spheroids had the highest levels of αSMA, collagen-1 and collagen-3 deposition among all groups as well as formation of a dense collagen rim surrounding the spheroid. Future studies exploring the greater fibrotic activity of F16 spheroids may provide new mechanistic insights into diseases involving excessive fibrotic activity. Microtissue fibrosis models capable of achieving greater clinical fidelity have the potential to combine the relevance of animal models with the scale, cost and throughput of in vitro testing.
中文翻译:

人成纤维细胞-巨噬细胞组织球体显示体外成纤维模型发展的比例依赖性纤维化活性。
纤维化是过量胶原蛋白的病理积累,是许多最常见疾病的基础,代表正常组织愈合的基本过程功能障碍。纤维化研究旨在限制这种反应,而不会改善纤维发生在器官功能中的重要作用。但是,缺乏实际的体外模型阻碍了对机制和潜在干预措施的研究,因为成纤维细胞的标准2D单层培养具有有限的适用性。我们寻求开发和优化纤维化球体:一种无支架的三维人成纤维细胞-巨噬细胞球体系统,代表了人类纤维化的改良台式模型。我们创建,表征并优化了仅人类成纤维细胞的球状体,证明与单层成纤维细胞相比,胶原蛋白沉积增加,而大于300μm的球体则逐渐增加凋亡。接下来,我们通过添加人类巨噬细胞来改进球体系统,以更精确地概括纤维生成过程中的环境,从而创建了一种混合的球体系统,其成纤维细胞和巨噬细胞比例为2:1至64:1。球状体(特别是16:1 [F16]比率)被激活的成纤维细胞更多,巨噬细胞极化朝促炎性M1表型发展。含有较大比例的巨噬细胞的杂化球体显示出较大的巨噬细胞异质性和较少的纤维生成,而较低的巨噬细胞比率限制了巨噬细胞诱导的作用并产生较少的胶原蛋白沉积。F16组的纤维化相关基因(Col-1a1,Col-3a1和TGF-β)和炎症相关基因(TNF,IL1β和IL6)。IF染色表明F16球体在所有组中具有最高水平的αSMA,胶原蛋白-1和胶原蛋白3沉积,以及围绕球体的致密胶原蛋白边缘的形成。未来研究探索更大的F16球状体纤维化活性的研究可能会为涉及过度纤维化活性的疾病提供新的机制。能够实现更高临床保真度的微组织纤维化模型具有将动物模型的相关性与体外测试的规模,成本和通量相结合的潜力。所有组中的胶原蛋白-1和胶原蛋白3沉积,以及围绕球体的致密胶原蛋白边缘的形成。未来研究探索更大的F16球状体纤维化活性的研究可能会为涉及过度纤维化活性的疾病提供新的机制。能够实现更高临床保真度的微组织纤维化模型具有将动物模型的相关性与体外测试的规模,成本和通量相结合的潜力。所有组中的胶原蛋白-1和胶原蛋白3沉积,以及围绕球体的致密胶原蛋白边缘的形成。未来研究探索更大的F16球状体纤维化活性的研究可能会为涉及过度纤维化活性的疾病提供新的机制。能够实现更高临床保真度的微组织纤维化模型具有将动物模型的相关性与体外测试的规模,成本和通量相结合的潜力。
更新日期:2020-02-14
中文翻译:

人成纤维细胞-巨噬细胞组织球体显示体外成纤维模型发展的比例依赖性纤维化活性。
纤维化是过量胶原蛋白的病理积累,是许多最常见疾病的基础,代表正常组织愈合的基本过程功能障碍。纤维化研究旨在限制这种反应,而不会改善纤维发生在器官功能中的重要作用。但是,缺乏实际的体外模型阻碍了对机制和潜在干预措施的研究,因为成纤维细胞的标准2D单层培养具有有限的适用性。我们寻求开发和优化纤维化球体:一种无支架的三维人成纤维细胞-巨噬细胞球体系统,代表了人类纤维化的改良台式模型。我们创建,表征并优化了仅人类成纤维细胞的球状体,证明与单层成纤维细胞相比,胶原蛋白沉积增加,而大于300μm的球体则逐渐增加凋亡。接下来,我们通过添加人类巨噬细胞来改进球体系统,以更精确地概括纤维生成过程中的环境,从而创建了一种混合的球体系统,其成纤维细胞和巨噬细胞比例为2:1至64:1。球状体(特别是16:1 [F16]比率)被激活的成纤维细胞更多,巨噬细胞极化朝促炎性M1表型发展。含有较大比例的巨噬细胞的杂化球体显示出较大的巨噬细胞异质性和较少的纤维生成,而较低的巨噬细胞比率限制了巨噬细胞诱导的作用并产生较少的胶原蛋白沉积。F16组的纤维化相关基因(Col-1a1,Col-3a1和TGF-β)和炎症相关基因(TNF,IL1β和IL6)。IF染色表明F16球体在所有组中具有最高水平的αSMA,胶原蛋白-1和胶原蛋白3沉积,以及围绕球体的致密胶原蛋白边缘的形成。未来研究探索更大的F16球状体纤维化活性的研究可能会为涉及过度纤维化活性的疾病提供新的机制。能够实现更高临床保真度的微组织纤维化模型具有将动物模型的相关性与体外测试的规模,成本和通量相结合的潜力。所有组中的胶原蛋白-1和胶原蛋白3沉积,以及围绕球体的致密胶原蛋白边缘的形成。未来研究探索更大的F16球状体纤维化活性的研究可能会为涉及过度纤维化活性的疾病提供新的机制。能够实现更高临床保真度的微组织纤维化模型具有将动物模型的相关性与体外测试的规模,成本和通量相结合的潜力。所有组中的胶原蛋白-1和胶原蛋白3沉积,以及围绕球体的致密胶原蛋白边缘的形成。未来研究探索更大的F16球状体纤维化活性的研究可能会为涉及过度纤维化活性的疾病提供新的机制。能够实现更高临床保真度的微组织纤维化模型具有将动物模型的相关性与体外测试的规模,成本和通量相结合的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号