Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Skeletal loading regulates breast cancer-associated osteolysis in a loading intensity-dependent fashion.
Bone Research ( IF 14.3 ) Pub Date : 2020-02-14 , DOI: 10.1038/s41413-020-0083-6 Yao Fan 1, 2 , Aydin Jalali 2 , Andy Chen 2 , Xinyu Zhao 2, 3 , Shengzhi Liu 2 , Meghana Teli 2 , Yunxia Guo 1, 2 , Fangjia Li 4 , Junrui Li 5 , Amanda Siegel 6 , Lianxiang Yang 5 , Jing Liu 4 , Sungsoo Na 2 , Mangilal Agarwal 6 , Alexander G Robling 7, 8 , Harikrishna Nakshatri 9 , Bai-Yan Li 1 , Hiroki Yokota 1, 2, 5, 6, 7, 8
Bone Research ( IF 14.3 ) Pub Date : 2020-02-14 , DOI: 10.1038/s41413-020-0083-6 Yao Fan 1, 2 , Aydin Jalali 2 , Andy Chen 2 , Xinyu Zhao 2, 3 , Shengzhi Liu 2 , Meghana Teli 2 , Yunxia Guo 1, 2 , Fangjia Li 4 , Junrui Li 5 , Amanda Siegel 6 , Lianxiang Yang 5 , Jing Liu 4 , Sungsoo Na 2 , Mangilal Agarwal 6 , Alexander G Robling 7, 8 , Harikrishna Nakshatri 9 , Bai-Yan Li 1 , Hiroki Yokota 1, 2, 5, 6, 7, 8
Affiliation
|
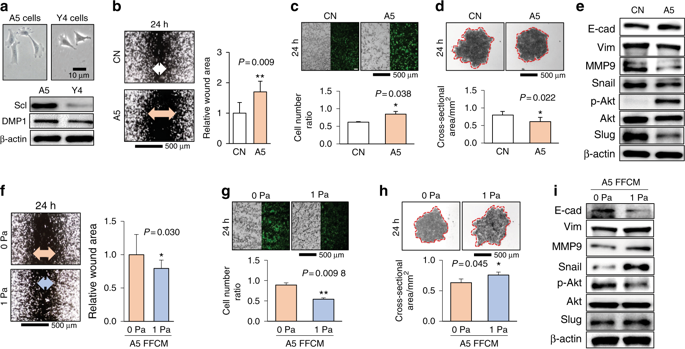
Osteocytes are mechanosensitive bone cells, but little is known about their effects on tumor cells in response to mechanical stimulation. We treated breast cancer cells with osteocyte-derived conditioned medium (CM) and fluid flow-treated conditioned medium (FFCM) with 0.25 Pa and 1 Pa shear stress. Notably, CM and FFCM at 0.25 Pa induced the mesenchymal-to-epithelial transition (MET), but FFCM at 1 Pa induced the epithelial-to-mesenchymal transition (EMT). This suggested that the effects of fluid flow on conditioned media depend on flow intensity. Fluorescence resonance energy transfer (FRET)-based evaluation of Src activity and vinculin molecular force showed that osteopontin was involved in EMT and MET switching. A mouse model of tumor-induced osteolysis was tested using dynamic tibia loadings of 1, 2, and 5 N. The low 1 N loading suppressed tumor-induced osteolysis, but this beneficial effect was lost and reversed with loads at 2 and 5 N, respectively. Changing the loading intensities in vivo also led to changes in serum TGFβ levels and the composition of tumor-associated volatile organic compounds in the urine. Collectively, this study demonstrated the critical role of intensity-dependent mechanotransduction and osteopontin in tumor-osteocyte communication, indicating that a biophysical factor can tangibly alter the behaviors of tumor cells in the bone microenvironment.
中文翻译:

骨骼负荷以负荷强度依赖性的方式调节与乳腺癌相关的骨溶解。
骨细胞是机械敏感的骨细胞,但人们对其响应机械刺激对肿瘤细胞的影响知之甚少。我们用骨细胞衍生的条件培养基(CM)和流体流动处理的条件培养基(FFCM)分别以0.25 Pa和1 Pa的剪切应力处理了乳腺癌细胞。值得注意的是,在0.25 Pa的CM和FFCM诱导了间质到上皮的转变(MET),但是在1 Pa的FFCM诱导了上皮到上皮的转变(EMT)。这表明流体流动对条件介质的影响取决于流动强度。基于荧光共振能量转移(FRET)的Src活性和纽蛋白分子力的评估表明,骨桥蛋白参与了EMT和MET的转换。使用1 N,2 N和5 N的动态胫骨载荷测试了小鼠肿瘤诱发的骨溶解的模型。1 N的低负荷抑制了肿瘤引起的骨溶解,但是这种有益的作用在2 N和5 N的负荷下分别消失和逆转。体内负荷强度的改变还导致血清TGFβ水平的改变以及尿液中与肿瘤相关的挥发性有机化合物的组成变化。集体,这项研究表明强度依赖的机械转导和骨桥蛋白在肿瘤-骨细胞通讯中的关键作用,表明生物物理因素可以切实改变骨微环境中肿瘤细胞的行为。
更新日期:2020-02-14
中文翻译:

骨骼负荷以负荷强度依赖性的方式调节与乳腺癌相关的骨溶解。
骨细胞是机械敏感的骨细胞,但人们对其响应机械刺激对肿瘤细胞的影响知之甚少。我们用骨细胞衍生的条件培养基(CM)和流体流动处理的条件培养基(FFCM)分别以0.25 Pa和1 Pa的剪切应力处理了乳腺癌细胞。值得注意的是,在0.25 Pa的CM和FFCM诱导了间质到上皮的转变(MET),但是在1 Pa的FFCM诱导了上皮到上皮的转变(EMT)。这表明流体流动对条件介质的影响取决于流动强度。基于荧光共振能量转移(FRET)的Src活性和纽蛋白分子力的评估表明,骨桥蛋白参与了EMT和MET的转换。使用1 N,2 N和5 N的动态胫骨载荷测试了小鼠肿瘤诱发的骨溶解的模型。1 N的低负荷抑制了肿瘤引起的骨溶解,但是这种有益的作用在2 N和5 N的负荷下分别消失和逆转。体内负荷强度的改变还导致血清TGFβ水平的改变以及尿液中与肿瘤相关的挥发性有机化合物的组成变化。集体,这项研究表明强度依赖的机械转导和骨桥蛋白在肿瘤-骨细胞通讯中的关键作用,表明生物物理因素可以切实改变骨微环境中肿瘤细胞的行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号