当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Suppressing hydrogen peroxide generation to achieve oxygen-insensitivity of a [NiFe] hydrogenase in redox active films.
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-14 , DOI: 10.1038/s41467-020-14673-7 Huaiguang Li 1 , Ute Münchberg 2 , Alaa A Oughli 1 , Darren Buesen 1 , Wolfgang Lubitz 3 , Erik Freier 2 , Nicolas Plumeré 1
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-14 , DOI: 10.1038/s41467-020-14673-7 Huaiguang Li 1 , Ute Münchberg 2 , Alaa A Oughli 1 , Darren Buesen 1 , Wolfgang Lubitz 3 , Erik Freier 2 , Nicolas Plumeré 1
Affiliation
|
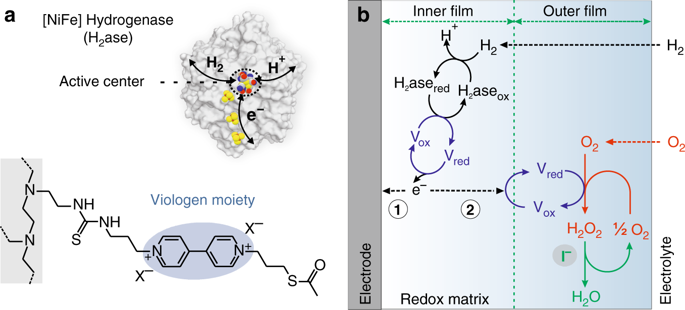
Redox-active films were proposed as protective matrices for preventing oxidative deactivation of oxygen-sensitive catalysts such as hydrogenases for their use in fuel cells. However, the theoretical models predict quasi-infinite protection from oxygen and the aerobic half-life for hydrogenase-catalyzed hydrogen oxidation within redox films lasts only about a day. Here, we employ operando confocal microscopy to elucidate the deactivation processes. The hydrogen peroxide generated from incomplete reduction of oxygen induces the decomposition of the redox matrix rather than deactivation of the biocatalyst. We show that efficient dismutation of hydrogen peroxide by iodide extends the aerobic half-life of the catalytic film containing an oxygen-sensitive [NiFe] hydrogenase to over one week, approaching the experimental anaerobic half-life. Altogether, our data support the theory that redox films make the hydrogenases immune against the direct deactivation by oxygen and highlight the importance of suppressing hydrogen peroxide production in order to reach complete protection from oxidative stress.
中文翻译:

抑制过氧化氢的产生以实现氧化还原活性膜中[NiFe]氢化酶的氧不敏感性。
氧化还原活性薄膜被提议作为保护基质,以防止氧敏感催化剂(例如燃料电池中使用的氢化酶)氧化失活。然而,理论模型预测,氧化还原膜内氢化酶催化的氢气氧化的有氧半衰期几乎是无限的,仅持续大约一天。在这里,我们采用操作共聚焦显微镜来阐明失活过程。氧气不完全还原产生的过氧化氢会引起氧化还原基质的分解,而不是使生物催化剂失活。我们表明,碘化物对过氧化氢的有效歧化将含有氧敏感[NiFe]氢化酶的催化膜的有氧半衰期延长至一周以上,接近实验的无氧半衰期。总而言之,我们的数据支持这样的理论:氧化还原膜使氢化酶免受氧气的直接失活,并强调了抑制过氧化氢产生以实现完全免受氧化应激的重要性。
更新日期:2020-02-14
中文翻译:

抑制过氧化氢的产生以实现氧化还原活性膜中[NiFe]氢化酶的氧不敏感性。
氧化还原活性薄膜被提议作为保护基质,以防止氧敏感催化剂(例如燃料电池中使用的氢化酶)氧化失活。然而,理论模型预测,氧化还原膜内氢化酶催化的氢气氧化的有氧半衰期几乎是无限的,仅持续大约一天。在这里,我们采用操作共聚焦显微镜来阐明失活过程。氧气不完全还原产生的过氧化氢会引起氧化还原基质的分解,而不是使生物催化剂失活。我们表明,碘化物对过氧化氢的有效歧化将含有氧敏感[NiFe]氢化酶的催化膜的有氧半衰期延长至一周以上,接近实验的无氧半衰期。总而言之,我们的数据支持这样的理论:氧化还原膜使氢化酶免受氧气的直接失活,并强调了抑制过氧化氢产生以实现完全免受氧化应激的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号