当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural insights into NDH-1 mediated cyclic electron transfer.
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-14 , DOI: 10.1038/s41467-020-14732-z Chunli Zhang 1, 2, 3 , Jin Shuai 4, 5 , Zhaoxing Ran 6 , Jiaohong Zhao 6 , Zhenfang Wu 1, 2 , Rijing Liao 1, 2 , Jian Wu 1, 2, 7 , Weimin Ma 6, 8 , Ming Lei 1, 2, 9
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-14 , DOI: 10.1038/s41467-020-14732-z Chunli Zhang 1, 2, 3 , Jin Shuai 4, 5 , Zhaoxing Ran 6 , Jiaohong Zhao 6 , Zhenfang Wu 1, 2 , Rijing Liao 1, 2 , Jian Wu 1, 2, 7 , Weimin Ma 6, 8 , Ming Lei 1, 2, 9
Affiliation
|
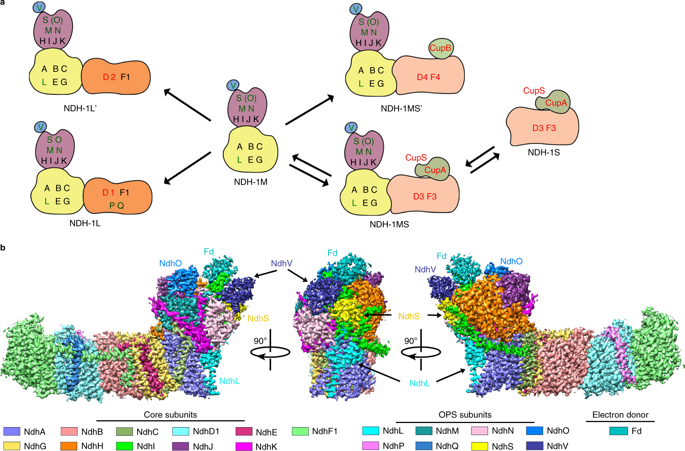
NDH-1 is a key component of the cyclic-electron-transfer around photosystem I (PSI CET) pathway, an important antioxidant mechanism for efficient photosynthesis. Here, we report a 3.2-Å-resolution cryo-EM structure of the ferredoxin (Fd)-NDH-1L complex from the cyanobacterium Thermosynechococcus elongatus. The structure reveals three β-carotene and fifteen lipid molecules in the membrane arm of NDH-1L. Regulatory oxygenic photosynthesis-specific (OPS) subunits NdhV, NdhS and NdhO are close to the Fd-binding site whilst NdhL is adjacent to the plastoquinone (PQ) cavity, and they play different roles in PSI CET under high-light stress. NdhV assists in the binding of Fd to NDH-1L and accelerates PSI CET in response to short-term high-light exposure. In contrast, prolonged high-light irradiation switches on the expression and assembly of the NDH-1MS complex, which likely contains no NdhO to further accelerate PSI CET and reduce ROS production. We propose that this hierarchical mechanism is necessary for the survival of cyanobacteria in an aerobic environment.
中文翻译:

NDH-1介导的循环电子转移的结构见解。
NDH-1是围绕光系统I(PSI CET)途径进行循环电子转移的关键成分,这是有效进行光合作用的重要抗氧化剂机制。在这里,我们报告了从蓝藻嗜热球菌的铁氧还蛋白(Fd)-NDH-1L复合物的3.2-Å分辨率的冷冻EM结构。该结构揭示了NDH-1L膜臂中的三个β-胡萝卜素和15个脂质分子。调节性氧合光合作用特异性(OPS)亚基NdhV,NdhS和NdhO接近Fd结合位点,而NdhL邻近质体醌(PQ)腔,在强光胁迫下它们在PSI CET中扮演不同的角色。NdhV有助于Fd与NDH-1L结合并响应短期强光照射而加速PSI CET。相反,长时间的强光照射会打开NDH-1MS复合物的表达和装配,该复合物可能不含NdhO,从而进一步加速PSI CET并降低ROS的产生。我们建议这种有层次的机制对于有氧环境中的蓝细菌的生存是必要的。
更新日期:2020-02-14
中文翻译:

NDH-1介导的循环电子转移的结构见解。
NDH-1是围绕光系统I(PSI CET)途径进行循环电子转移的关键成分,这是有效进行光合作用的重要抗氧化剂机制。在这里,我们报告了从蓝藻嗜热球菌的铁氧还蛋白(Fd)-NDH-1L复合物的3.2-Å分辨率的冷冻EM结构。该结构揭示了NDH-1L膜臂中的三个β-胡萝卜素和15个脂质分子。调节性氧合光合作用特异性(OPS)亚基NdhV,NdhS和NdhO接近Fd结合位点,而NdhL邻近质体醌(PQ)腔,在强光胁迫下它们在PSI CET中扮演不同的角色。NdhV有助于Fd与NDH-1L结合并响应短期强光照射而加速PSI CET。相反,长时间的强光照射会打开NDH-1MS复合物的表达和装配,该复合物可能不含NdhO,从而进一步加速PSI CET并降低ROS的产生。我们建议这种有层次的机制对于有氧环境中的蓝细菌的生存是必要的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号