当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into pressure tunable reaction rates for electrochemical reduction of CO2 in organic electrolytes
Green Chemistry ( IF 9.3 ) Pub Date : 2020-02-13 , DOI: 10.1039/d0gc00013b Charles I. Shaughnessy 1, 2, 3, 4, 5 , David J. Sconyers 1, 2, 3, 4, 6 , Hyun-Jin Lee 1, 2, 3, 4 , Bala Subramaniam 1, 2, 3, 4, 5 , James D. Blakemore 1, 2, 3, 4, 6 , Kevin C. Leonard 1, 2, 3, 4, 5
Green Chemistry ( IF 9.3 ) Pub Date : 2020-02-13 , DOI: 10.1039/d0gc00013b Charles I. Shaughnessy 1, 2, 3, 4, 5 , David J. Sconyers 1, 2, 3, 4, 6 , Hyun-Jin Lee 1, 2, 3, 4 , Bala Subramaniam 1, 2, 3, 4, 5 , James D. Blakemore 1, 2, 3, 4, 6 , Kevin C. Leonard 1, 2, 3, 4, 5
Affiliation
|
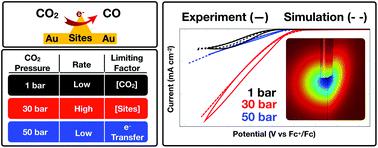
Electrochemical CO2 reduction rates are limited in aqueous-based electrolytes by the low solubility of CO2. We recently demonstrated that organic solvent-based CO2 Expanded Electrolytes (CXEs) can solubilize multi-molar amounts of CO2 at moderate pressures while retaining sufficient supporting electrolyte to facilitate electrochemistry. Up to an order of magnitude enhancement in CO2 reduction rates to CO was achieved on polycrystalline Au electrodes at faradaic efficiencies approaching 80%. Herein, we show that similar enhancements are observed on Cu catalysts as well, on the basis of enhanced current flow. On both systems, a maximum in CO2 reduction rate was observed at ca. 5 M CO2 concentration (i.e., 3.1 MPa head-space pressure) beyond which the rate decreases. To explain this counterintuitive phenomenon, we developed a detailed COMSOL-based mechanistic model of CO2 reduction in organic electrolytes under elevated pressures on Au electrodes. This model incorporates the dissimilar variations of the key physicochemical properties (viz., CO2 concentration, CO2 diffusion rate, and solution polarity) with CO2 pressure. We thereby demonstrate that the overall rate is limited by CO2 concentrations at lower than optimum CO2 pressures. At pressures higher than the optimum the rate is limited by both an attenuation of the first electron-transfer step and an increase in the ohmic resistance of the system. Excellent quantitative match between experimental and model-predicted rates lend credence to the proposed underlying mechanism of electrocatalytic CO2 reduction in CXEs. These fundamental insights provide guidance for the rational design and scaleup of highly efficient CXE-based CO2 reduction systems.
中文翻译:

洞察压力可调反应速率以电化学还原有机电解质中的CO2
在水基电解质中,由于CO 2的溶解度低,电化学的CO 2还原速率受到限制。最近,我们证明了基于有机溶剂的CO 2膨胀电解质(CXE)可以在中等压力下溶解多摩尔量的CO 2,同时保留足够的支持电解质以促进电化学。在法拉第效率接近80%的多晶Au电极上,CO 2还原成CO的速率最多提高了一个数量级。在本文中,我们表明,基于增强的电流,在铜催化剂上也观察到了类似的增强。在两个系统上,均观察到最大的CO 2还原速率为ca. 5 M CO 2浓度(即3.1 MPa顶空压力),超过该浓度速率会降低。为了解释这种违反直觉的现象,我们开发了基于COMSOL的详细模型,该模型在Au电极上在高压下有机电解质中CO 2还原的机理。该模型结合了关键物理化学性质(即,CO 2浓度,CO 2扩散速率和溶液极性)随CO 2压力的不同变化。因此,我们证明了总速率受低于最佳CO 2的CO 2浓度的限制压力。在高于最佳压力的压力下,速率受到第一电子转移步骤的衰减和系统欧姆电阻的增加的限制。实验速率与模型预测速率之间的出色定量匹配为所提出的CXE中电催化还原CO 2的潜在机理提供了依据。这些基本见解为高效设计基于CXE的CO 2还原系统的合理设计和规模扩展提供了指导。
更新日期:2020-02-13
中文翻译:

洞察压力可调反应速率以电化学还原有机电解质中的CO2
在水基电解质中,由于CO 2的溶解度低,电化学的CO 2还原速率受到限制。最近,我们证明了基于有机溶剂的CO 2膨胀电解质(CXE)可以在中等压力下溶解多摩尔量的CO 2,同时保留足够的支持电解质以促进电化学。在法拉第效率接近80%的多晶Au电极上,CO 2还原成CO的速率最多提高了一个数量级。在本文中,我们表明,基于增强的电流,在铜催化剂上也观察到了类似的增强。在两个系统上,均观察到最大的CO 2还原速率为ca. 5 M CO 2浓度(即3.1 MPa顶空压力),超过该浓度速率会降低。为了解释这种违反直觉的现象,我们开发了基于COMSOL的详细模型,该模型在Au电极上在高压下有机电解质中CO 2还原的机理。该模型结合了关键物理化学性质(即,CO 2浓度,CO 2扩散速率和溶液极性)随CO 2压力的不同变化。因此,我们证明了总速率受低于最佳CO 2的CO 2浓度的限制压力。在高于最佳压力的压力下,速率受到第一电子转移步骤的衰减和系统欧姆电阻的增加的限制。实验速率与模型预测速率之间的出色定量匹配为所提出的CXE中电催化还原CO 2的潜在机理提供了依据。这些基本见解为高效设计基于CXE的CO 2还原系统的合理设计和规模扩展提供了指导。










































 京公网安备 11010802027423号
京公网安备 11010802027423号