当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The adsorption and oxidation of SO2 on MgO surface: experimental and DFT calculation studies
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-02-13 , DOI: 10.1039/c9en01474h Honghong Wang 1, 2, 3, 4, 5 , Cheng Zhong 1, 2, 3, 4, 5 , Qingxin Ma 1, 2, 3, 4, 5 , Jinzhu Ma 1, 2, 3, 4, 5 , Hong He 1, 2, 3, 4, 5
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-02-13 , DOI: 10.1039/c9en01474h Honghong Wang 1, 2, 3, 4, 5 , Cheng Zhong 1, 2, 3, 4, 5 , Qingxin Ma 1, 2, 3, 4, 5 , Jinzhu Ma 1, 2, 3, 4, 5 , Hong He 1, 2, 3, 4, 5
Affiliation
|
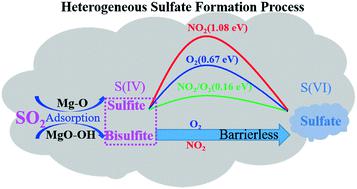
The heterogeneous oxidation of sulfur dioxide (SO2) to sulfate on the surface of MgO particles was investigated by in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and density functional theory (DFT). The in situ DRIFTS spectra show that the major products of SO2 adsorption on MgO particles are sulfite, bisulfite and sulfate, while the coexisting NO2 and O2 promote the conversion of sulfite/bisulfite into sulfate. DFT calculations show that the main adsorption products of SO2 on the perfect MgO (001) surface, the hydroxylated MgO (001) surface and the step sites of the MgO (001) surface are sulfite (SO2,gas + Olattice → SO3,ads), bisulfite and sulfate, respectively. The oxidation of sulfite to sulfate was hardly promoted by the presence of O2 at room temperature, because the process needs to overcome an energy barrier of 0.67 eV. The existence of NO2 couldn't promote the formation of sulfate, because the direct oxidation of sulfite into sulfate by NO2 is difficult (ΔEa = 1.08 eV). However, coexisting NO2 with O2 can facilitate the oxidation of SO2, which illustrates the micro-mechanism of the synergistic effect between SO2 and NO2 on MgO. In contrast to sulfite, surface bisulfite formed through the adsorption of SO2 on surface OH could be oxidized by O2 or NO2via barrierless processes. This mechanism highlights the synergistic effects of O2/NO2 as well as surface hydroxyl on the heterogeneous oxidation of SO2 on the MgO surface. Atmospheric implications of the heterogeneous oxidation of SO2 on MgO under ambient atmosphere are discussed.
中文翻译:

SO2在MgO表面的吸附和氧化:实验和DFT计算研究
利用原位漫反射红外傅里叶变换光谱法(DRIFTS)和密度泛函理论(DFT)研究了MgO颗粒表面二氧化硫(SO 2)向硫酸盐的非均相氧化。在原位DRIFTS谱图表明,SO的主要产物2吸附对MgO颗粒是亚硫酸盐,亚硫酸氢盐和硫酸盐,而共存的NO 2和O 2促进亚硫酸盐/亚硫酸氢盐的成硫酸盐的转化率。DFT计算表明,在理想的MgO(001)表面,羟基化的MgO(001)表面和MgO(001)表面的台阶处,SO 2的主要吸附产物为亚硫酸盐(SO 2+ O晶格→SO 3,ads),亚硫酸氢盐和硫酸盐。在室温下,O 2的存在几乎不会促进亚硫酸盐氧化为硫酸盐,因为该过程需要克服0.67 eV的能垒。NO 2的存在不能促进硫酸盐的形成,因为亚硝酸盐很难被NO 2直接氧化成硫酸盐(ΔE a = 1.08 eV)。然而,NO 2与O 2共存可以促进SO 2的氧化,这说明了SO 2与NO 2协同作用的微观机制。在MgO上。与亚硫酸盐相反,通过SO 2吸附在表面OH上形成的表面亚硫酸氢盐可以通过无障碍工艺被O 2或NO 2氧化。这种机理突出了O 2 / NO 2以及表面羟基对MgO表面SO 2异相氧化的协同作用。讨论了大气环境下MgO上SO 2异质氧化的大气影响。
更新日期:2020-02-13
中文翻译:

SO2在MgO表面的吸附和氧化:实验和DFT计算研究
利用原位漫反射红外傅里叶变换光谱法(DRIFTS)和密度泛函理论(DFT)研究了MgO颗粒表面二氧化硫(SO 2)向硫酸盐的非均相氧化。在原位DRIFTS谱图表明,SO的主要产物2吸附对MgO颗粒是亚硫酸盐,亚硫酸氢盐和硫酸盐,而共存的NO 2和O 2促进亚硫酸盐/亚硫酸氢盐的成硫酸盐的转化率。DFT计算表明,在理想的MgO(001)表面,羟基化的MgO(001)表面和MgO(001)表面的台阶处,SO 2的主要吸附产物为亚硫酸盐(SO 2+ O晶格→SO 3,ads),亚硫酸氢盐和硫酸盐。在室温下,O 2的存在几乎不会促进亚硫酸盐氧化为硫酸盐,因为该过程需要克服0.67 eV的能垒。NO 2的存在不能促进硫酸盐的形成,因为亚硝酸盐很难被NO 2直接氧化成硫酸盐(ΔE a = 1.08 eV)。然而,NO 2与O 2共存可以促进SO 2的氧化,这说明了SO 2与NO 2协同作用的微观机制。在MgO上。与亚硫酸盐相反,通过SO 2吸附在表面OH上形成的表面亚硫酸氢盐可以通过无障碍工艺被O 2或NO 2氧化。这种机理突出了O 2 / NO 2以及表面羟基对MgO表面SO 2异相氧化的协同作用。讨论了大气环境下MgO上SO 2异质氧化的大气影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号