当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ruthenium‐Catalyzed Double C(sp2)−H Functionalizations of Fumaramides with Alkynes for the Divergent Synthesis of Pyridones and Naphthyridinediones
ChemCatChem ( IF 3.8 ) Pub Date : 2020-03-23 , DOI: 10.1002/cctc.201902160 Zhi‐Jian Han 1 , Ze‐Xuan Zhang 2 , Wei‐Ping Li 2, 3 , Zhi‐Hong Du 2 , Bao‐Xiu Tao 2 , Chao‐Shan Da 2 , Zuo‐Yi Jiao 1 , Hao Chen 1 , Yumin Li 1
ChemCatChem ( IF 3.8 ) Pub Date : 2020-03-23 , DOI: 10.1002/cctc.201902160 Zhi‐Jian Han 1 , Ze‐Xuan Zhang 2 , Wei‐Ping Li 2, 3 , Zhi‐Hong Du 2 , Bao‐Xiu Tao 2 , Chao‐Shan Da 2 , Zuo‐Yi Jiao 1 , Hao Chen 1 , Yumin Li 1
Affiliation
|
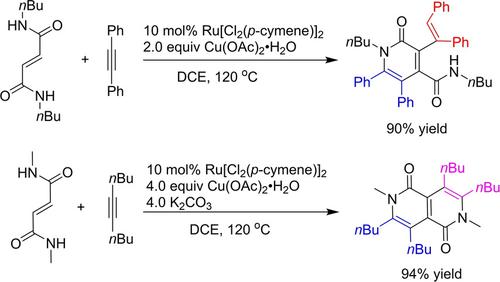
Transition‐metal‐catalyzed C−H functionalization of aromatic secondary amides with alkynes mainly undergo C−H/N−H annulation but rarely undergo ortho‐alkenylation. It is particularly challenging to selectively realize both oxidative annulation and ortho‐alkenylation of aromatic secondary amides with alkynes in the transition‐metal‐catalysis. In this article, we synthesized fully‐substituted 2‐pyridones and 2,6‐naphthyridine‐1,5‐diones via C(sp2)−H functionalization of fumaramides for the first time. Under the Ru‐catalysis, fumaramides and 1,2‐diaryl ethynes first undergo C−H/N−H annulation leading to the intermediate 2‐pyridone with an exocyclic secondary amide, and subsequently undergo the unexpected stereoselective C−H alkenylation to realize fully substituted 2‐pyridones bearing an exocyclic anti alkenyl group. On the addition of K2CO3, however, the transformation of fumaramides with 1,2‐dialkyl ethyne undergoes two conventional C−H/N−H annulations to provide 2,6‐naphthyridine‐1,5‐diones in high yield. The two procedures can be successfully enlarged to gram‐scales without erosion of the yields. In addition, some 2‐pyridones and 2,6‐naphthyridine‐1,5‐diones emitting clear ultraviolent and fluorescent light, indicating the potential utility of this work in organic light‐emitting materials.
中文翻译:

富马酰胺和炔烃的钌催化双C(sp2)-H官能化,用于吡啶酮和萘啶酮的不同合成
带有炔烃的芳族仲酰胺的过渡金属催化CH功能化主要经历CH / NH环化反应,但很少经历邻烯基化反应。在过渡金属催化中,选择性地同时实现芳族仲酰胺的氧化环化和芳族仲酰胺的邻烯基化特别具有挑战性。在本文中,我们通过C(sp 2合成了完全取代的2-吡啶酮和2,6-萘啶-1,5-二酮)-富马酰胺的H官能化首次。在Ru催化下,富马酰胺和1,2-二芳基乙炔首先经历CH / NH环化反应,生成带有环外仲酰胺的中间2-吡啶酮,然后经历意想不到的立体选择性CH烯基化,以实现完全带有环外反烯基的取代2-吡啶酮。关于K 2 CO 3的添加但是,用1,2-二烷基乙炔进行的富马酰胺的转化经历了两次常规的CH / NH环化反应,从而以高收率提供了2,6-萘啶-1,5-二酮。可以成功地将这两个过程放大到克级,而不会影响产量。另外,一些2-吡啶酮和2,6-萘啶-1,5-二酮发出清晰的紫外线和荧光,表明这项工作在有机发光材料中的潜在用途。
更新日期:2020-03-23
中文翻译:

富马酰胺和炔烃的钌催化双C(sp2)-H官能化,用于吡啶酮和萘啶酮的不同合成
带有炔烃的芳族仲酰胺的过渡金属催化CH功能化主要经历CH / NH环化反应,但很少经历邻烯基化反应。在过渡金属催化中,选择性地同时实现芳族仲酰胺的氧化环化和芳族仲酰胺的邻烯基化特别具有挑战性。在本文中,我们通过C(sp 2合成了完全取代的2-吡啶酮和2,6-萘啶-1,5-二酮)-富马酰胺的H官能化首次。在Ru催化下,富马酰胺和1,2-二芳基乙炔首先经历CH / NH环化反应,生成带有环外仲酰胺的中间2-吡啶酮,然后经历意想不到的立体选择性CH烯基化,以实现完全带有环外反烯基的取代2-吡啶酮。关于K 2 CO 3的添加但是,用1,2-二烷基乙炔进行的富马酰胺的转化经历了两次常规的CH / NH环化反应,从而以高收率提供了2,6-萘啶-1,5-二酮。可以成功地将这两个过程放大到克级,而不会影响产量。另外,一些2-吡啶酮和2,6-萘啶-1,5-二酮发出清晰的紫外线和荧光,表明这项工作在有机发光材料中的潜在用途。











































 京公网安备 11010802027423号
京公网安备 11010802027423号