Journal of Perinatology ( IF 2.9 ) Pub Date : 2020-02-13 , DOI: 10.1038/s41372-020-0608-2 Mythily Sindhur 1 , Haribalakrishna Balasubramanian 1 , Lakshmi Srinivasan 2 , Nandkishor S Kabra 1 , Prachi Agashe 1 , Ashish Doshi 1
|
Objective
To study the efficacy of intranasal fentanyl as an adjunct for pain management during screening for retinopathy of prematurity (ROP) in preterm infants.
Study design
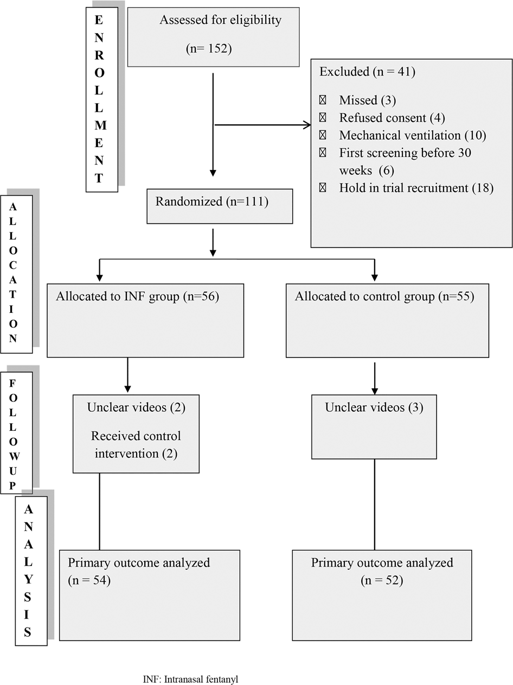
In this single center, double blinded, randomized controlled trial, preterm neonates between 30 and 34 weeks postmenstrual age received either intranasal fentanyl (2 mcg/kg) or intranasal normal saline through a mucosal atomization device 5 min prior to the first ROP-screening examination. Both the groups received standard pain relief strategies (oral sucrose, 0.5% proparacaine eye drops and physical containment). The primary outcome was premature infant pain profile-revised (PIPP-R) score during the screening.
Results
A total of 111 infants were enrolled. PIPP-R score during the retinal examination was significantly lower in the fentanyl group (8.3 versus 11.5, mean difference: 3.2 (2.46–4.06), P < 0.001). There was no significant difference in the incidence of adverse effects.
Conclusion
Intranasal fentanyl significantly reduced the pain associated with retinal examination without increasing the risk of respiratory depression. Large RCTs are required to verify the efficacy and safety of intranasal fentanyl for acute procedural pain in neonates.
Clinical Trial Registration
CTRI/2017/12/011016.
中文翻译:

早产儿早产儿视网膜病变筛查期间鼻内芬太尼用于疼痛管理:一项随机对照试验
客观的
研究鼻内芬太尼作为早产儿早产儿视网膜病变 (ROP) 筛查期间疼痛管理辅助手段的疗效。
学习规划
在这项单中心、双盲、随机对照试验中,月经后 30 至 34 周的早产儿在第一次 ROP 筛查检查前 5 分钟通过粘膜雾化装置接受鼻内芬太尼 (2 mcg/kg) 或鼻内生理盐水. 两组都接受了标准的疼痛缓解策略(口服蔗糖、0.5% 丙美卡因滴眼液和物理控制)。主要结果是筛查期间的早产儿疼痛特征修订 (PIPP-R) 评分。
结果
共有 111 名婴儿入组。芬太尼组视网膜检查期间的 PIPP-R 评分显着降低(8.3 对 11.5,平均差:3.2(2.46-4.06),P < 0.001)。不良反应的发生率没有显着差异。
结论
鼻内芬太尼可显着减轻与视网膜检查相关的疼痛,而不会增加呼吸抑制的风险。需要大型随机对照试验来验证鼻内芬太尼治疗新生儿急性操作性疼痛的有效性和安全性。
临床试验注册
CTRI/2017/12/011016。



























 京公网安备 11010802027423号
京公网安备 11010802027423号