Gastroenterology ( IF 25.7 ) Pub Date : 2020-02-12 , DOI: 10.1053/j.gastro.2020.02.011 Dan Li 1 , Nan Li 2 , Yi-Fan Zhang 2 , Haiying Fu 3 , Mingqian Feng 2 , Dina Schneider 4 , Ling Su 5 , Xiaolin Wu 5 , Jing Zhou 6 , Sean Mackay 6 , Josh Kramer 7 , Zhijian Duan 2 , Hongjia Yang 2 , Aarti Kolluri 2 , Alissa M Hummer 2 , Madeline B Torres 2 , Hu Zhu 8 , Matthew D Hall 8 , Xiaoling Luo 9 , Jinqiu Chen 9 , Qun Wang 10 , Daniel Abate-Daga 11 , Boro Dropulic 4 , Stephen M Hewitt 12 , Rimas J Orentas 13 , Tim F Greten 14 , Mitchell Ho 2
|
Background and Aims
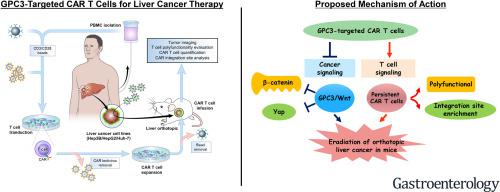
Glypican 3 (GPC3) is an oncofetal antigen involved in Wnt-dependent cell proliferation that is highly expressed in hepatocellular carcinoma (HCC). We investigated whether the functions of chimeric antigen receptors (CARs) that target GPC3 are affected by their antibody-binding properties.
Methods
We collected peripheral blood mononuclear cells from healthy donors and patients with HCC and used them to create CAR T cells, based on the humanized YP7 (hYP7) and HN3 antibodies, which have high affinities for the C-lobe and N-lobe of GPC3, respectively. NOD/SCID/IL-2Rgcnull (NSG) mice were given intraperitoneal injections of luciferase-expressing (Luc) Hep3B or HepG2 cells and after xenograft tumors formed, mice were given injections of saline or untransduced T cells (mock control), or CAR (HN3) T cells or CAR (hYP7) T cells. In other NOD/SCID/IL-2Rgcnull (NSG) mice, HepG2-Luc or Hep3B-Luc cells were injected into liver, and after orthotopic tumors formed, mice were given 1 injection of CAR (hYP7) T cells or CD19 CAR T cells (control). We developed droplet digital polymerase chain reaction and genome sequencing methods to analyze persistent CAR T cells in mice.
Results
Injections of CAR (hYP7) T cells eliminated tumors in 66% of mice by week 3, whereas CAR (HN3) T cells did not reduce tumor burden. Mice given CAR (hYP7) T cells remained tumor free after re-challenge with additional Hep3B cells. The CAR T cells induced perforin- and granzyme-mediated apoptosis and reduced levels of active β-catenin in HCC cells. Mice injected with CAR (hYP7) T cells had persistent expansion of T cells and subsets of polyfunctional CAR T cells via antigen-induced selection. These T cells were observed in the tumor microenvironment and spleen for up to 7 weeks after CAR T-cell administration. Integration sites in pre-infusion CAR (HN3) and CAR (hYP7) T cells were randomly distributed, whereas integration into NUPL1 was detected in 3.9% of CAR (hYP7) T cells 5 weeks after injection into tumor-bearing mice and 18.1% of CAR (hYP7) T cells at week 7. There was no common site of integration in CAR (HN3) or CD19 CAR T cells from tumor-bearing mice.
Conclusions
In mice with xenograft or orthoptic liver tumors, CAR (hYP7) T cells eliminate GPC3-positive HCC cells, possibly by inducing perforin- and granzyme-mediated apoptosis or reducing Wnt signaling in tumor cells. GPC3-targeted CAR T cells might be developed for treatment of patients with HCC.
中文翻译:

靶向磷脂酰肌醇蛋白聚糖 3 的持久多功能嵌合抗原受体 T 细胞可消除小鼠原位肝细胞癌。
背景和目标
磷脂酰肌醇蛋白聚糖 3 (GPC3) 是一种癌胚抗原,参与 Wnt 依赖性细胞增殖,在肝细胞癌 (HCC) 中高度表达。我们研究了靶向 GPC3 的嵌合抗原受体 (CAR) 的功能是否受到其抗体结合特性的影响。
方法
我们收集了健康捐献者和肝癌患者的外周血单核细胞,并用它们来创建基于人源化 YP7 (hYP7) 和 HN3 抗体的 CAR T 细胞,这些抗体与 GPC3 的 C 叶和 N 叶具有高亲和力,分别。 NOD/SCID/IL-2Rgc null (NSG) 小鼠腹腔注射荧光素酶表达 (Luc) Hep3B 或 HepG2 细胞,异种移植肿瘤形成后,小鼠注射盐水或未转导的 T 细胞(模拟对照)或 CAR (HN3) T 细胞或 CAR (hYP7) T 细胞。在其他 NOD/SCID/IL-2Rgc null (NSG) 小鼠中,将 HepG2-Luc 或 Hep3B-Luc 细胞注射到肝脏中,原位肿瘤形成后,给小鼠注射 1 次 CAR (hYP7) T 细胞或 CD19 CAR T细胞(对照)。我们开发了液滴数字聚合酶链式反应和基因组测序方法来分析小鼠体内的持久性 CAR T 细胞。
结果
注射 CAR (hYP7) T 细胞在第 3 周消除了 66% 的小鼠的肿瘤,而 CAR (HN3) T 细胞并没有减少肿瘤负荷。给予 CAR (hYP7) T 细胞的小鼠在用额外的 Hep3B 细胞重新攻击后仍保持无肿瘤状态。 CAR T 细胞诱导穿孔素和颗粒酶介导的细胞凋亡,并降低 HCC 细胞中活性 β-连环蛋白的水平。注射 CAR (hYP7) T 细胞的小鼠通过抗原诱导选择,T 细胞和多功能 CAR T 细胞亚群持续扩增。给予 CAR T 细胞后,这些 T 细胞在肿瘤微环境和脾脏中观察长达 7 周。输注前 CAR (HN3) 和 CAR (hYP7) T 细胞中的整合位点是随机分布的,而注射到荷瘤小鼠体内 5 周后,3.9% 的 CAR (hYP7) T 细胞检测到 NUPL1 整合,18.1% 的 CAR (hYP7) T 细胞被检测到整合到NUPL1中。第 7 周时的 CAR (hYP7) T 细胞。来自荷瘤小鼠的 CAR (HN3) 或 CD19 CAR T 细胞中没有共同的整合位点。
结论
在患有异种移植或正交肝肿瘤的小鼠中,CAR (hYP7) T 细胞可能通过诱导穿孔素和颗粒酶介导的细胞凋亡或减少肿瘤细胞中的 Wnt 信号传导来消除 GPC3 阳性 HCC 细胞。 GPC3 靶向 CAR T 细胞可能被开发用于治疗 HCC 患者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号