当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Friedel−Crafts‐Type Allylation of Phenol Derivatives Catalyzed by In Situ‐Generated Silyl Cyanometallates
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-03-03 , DOI: 10.1002/ajoc.202000023 Taiga Yurino 1 , Hamdiye Ece 2 , Takeshi Ohkuma 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-03-03 , DOI: 10.1002/ajoc.202000023 Taiga Yurino 1 , Hamdiye Ece 2 , Takeshi Ohkuma 1
Affiliation
|
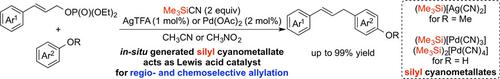
We successfully demonstrated that Friedel−Crafts‐type allylation of phenol derivatives and allylic phosphates is catalyzed by the AgTFA/trimethylsilyl cyanide (Me3SiCN) and Pd(OAc)2/Me3SiCN combined systems to afford the C‐allylated product in a highly regioselective manner. The corresponding silyl cyanometallates generated in situ are proposed to be the active catalytic species. Lewis acidity of the reversibly formed ion pairs is appropriately regulated for this reaction. The para‐allylated anisole and phenol derivatives are selectively obtained. The para‐substituted ones are converted to the ortho‐allylated products. The reactivity of the catalytic systems is strongly dependent on the electronic nature of both electrophile and nucleophile. Substitution of an aromatic ring on the allylic phosphate is essential for the reaction. Thus, the competitive reaction of a 1 : 1 mixture of cinnamyl and simple allyl phosphates affords only the cinnamyl‐substituted product.
中文翻译:

原位生成的氰基硅烷金属硅酸盐催化的苯酚衍生物的Friedel-Crafts型烯丙基化
我们成功地证明了苯酚衍生物和烯丙基磷酸盐的弗里德尔-克拉夫茨型烯丙基化由AgTFA /氰化三甲基硅烷(ME催化3的SiCN)和Pd(OAc)2 / Me的3的SiCN组合系统,得到Ç -allylated产物在高度区域选择性的方式。原位产生的相应的甲硅烷基氰基金属盐被认为是活性催化物质。可逆地形成的离子对的路易斯酸度对此反应进行适当调节。的对位被选择性地获得-allylated苯甲醚和苯酚衍生物。对位取代的被转换为邻位烯丙基化产品。催化体系的反应性在很大程度上取决于亲电试剂和亲核试剂的电子性质。芳基磷酸酯上芳环的取代对于反应是必不可少的。因此,肉桂酸和简单的烯丙基磷酸酯的1:1混合物的竞争性反应只能得到肉桂酸取代的产物。
更新日期:2020-04-21
中文翻译:

原位生成的氰基硅烷金属硅酸盐催化的苯酚衍生物的Friedel-Crafts型烯丙基化
我们成功地证明了苯酚衍生物和烯丙基磷酸盐的弗里德尔-克拉夫茨型烯丙基化由AgTFA /氰化三甲基硅烷(ME催化3的SiCN)和Pd(OAc)2 / Me的3的SiCN组合系统,得到Ç -allylated产物在高度区域选择性的方式。原位产生的相应的甲硅烷基氰基金属盐被认为是活性催化物质。可逆地形成的离子对的路易斯酸度对此反应进行适当调节。的对位被选择性地获得-allylated苯甲醚和苯酚衍生物。对位取代的被转换为邻位烯丙基化产品。催化体系的反应性在很大程度上取决于亲电试剂和亲核试剂的电子性质。芳基磷酸酯上芳环的取代对于反应是必不可少的。因此,肉桂酸和简单的烯丙基磷酸酯的1:1混合物的竞争性反应只能得到肉桂酸取代的产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号