当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Non‐Enzymatic Hybrid Catalysis for Stereoconversion of l‐Amino Acid Derivatives to d‐Isomers
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-02-20 , DOI: 10.1002/ajoc.202000067 Yuya Nagato 1 , Mari Kiyokawa 1 , Yusuke Ueki 1 , Jun Kikuchi 2 , Kohsuke Ohmatsu 1 , Masahiro Terada 2 , Takashi Ooi 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-02-20 , DOI: 10.1002/ajoc.202000067 Yuya Nagato 1 , Mari Kiyokawa 1 , Yusuke Ueki 1 , Jun Kikuchi 2 , Kohsuke Ohmatsu 1 , Masahiro Terada 2 , Takashi Ooi 1
Affiliation
|
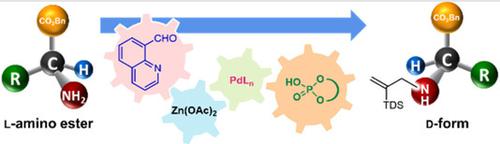
A catalytic transformation of N‐unprotected l‐amino esters to N‐protected d‐amino esters was developed. The combined use of a heteroaromatic aldehyde, Lewis acid, palladium complex, and chiral Brønsted acid was a key factor for the successful operation of this catalytic system. The synergistic cooperation of an appropriate aldehyde and Lewis acid was crucial for promoting an efficient racemization of l‐amino esters, while the combination of a palladium complex and chiral phosphoric acid enabled dynamic kinetic resolution through asymmetric N‐allylation, providing N‐protected d‐amino esters in good yields with up to 97 : 3 er. Following appropriate deprotection processes, d‐amino acids were obtained without loss of enantiomeric purity.
中文翻译:

非酶杂催化将l-氨基酸衍生物立体转化为d-异构体
的催化转化Ñ -unprotected升-氨基酯Ñ -protected d -氨基酯的开发。杂芳醛,路易斯酸,钯配合物和手性布朗斯台德酸的组合使用是该催化系统成功运行的关键因素。适当的醛和路易斯酸的协同作用对于促进l-氨基酯的高效外消旋作用至关重要,而钯配合物和手性磷酸的组合则可通过不对称N-烯丙基化实现动态动力学拆分,从而提供N保护的d氨基酯收率高达97:3 er。经过适当的脱保护过程,在不损失对映体纯度的情况下获得了d-氨基酸。
更新日期:2020-04-21
中文翻译:

非酶杂催化将l-氨基酸衍生物立体转化为d-异构体
的催化转化Ñ -unprotected升-氨基酯Ñ -protected d -氨基酯的开发。杂芳醛,路易斯酸,钯配合物和手性布朗斯台德酸的组合使用是该催化系统成功运行的关键因素。适当的醛和路易斯酸的协同作用对于促进l-氨基酯的高效外消旋作用至关重要,而钯配合物和手性磷酸的组合则可通过不对称N-烯丙基化实现动态动力学拆分,从而提供N保护的d氨基酯收率高达97:3 er。经过适当的脱保护过程,在不损失对映体纯度的情况下获得了d-氨基酸。











































 京公网安备 11010802027423号
京公网安备 11010802027423号