Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Introduction to a manuscript series on the characterization and use of microphysiological systems (MPS) in pharmaceutical safety and ADME applications.
Lab on a Chip ( IF 6.1 ) Pub Date : 2020-02-19 , DOI: 10.1039/c9lc01168d Kristin Fabre 1 , Brian Berridge 2 , William R Proctor 3 , Sherry Ralston 4 , Yvonne Will 5 , Szczepan W Baran 6 , Gorm Yoder 7 , Terry R Van Vleet 4
Lab on a Chip ( IF 6.1 ) Pub Date : 2020-02-19 , DOI: 10.1039/c9lc01168d Kristin Fabre 1 , Brian Berridge 2 , William R Proctor 3 , Sherry Ralston 4 , Yvonne Will 5 , Szczepan W Baran 6 , Gorm Yoder 7 , Terry R Van Vleet 4
Affiliation
|
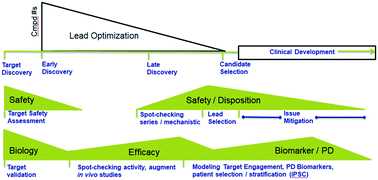
Safety related drug failures continue to be a challenge for pharmaceutical companies despite the numerous complex and lengthy in vitro assays and in vivo studies that make up the typical safety screening funnel. A lack of complete translation of animal data to humans can explain some of those shortcomings. Differences in sensitivity and drug disposition between animals and humans may also play a role. Many gaps exist for potential target tissues of drugs that cannot be adequately modeled in vitro. Microphysiological systems (MPS) may help to better model these target tissues and provide an opportunity to better assess some aspects of human safety prior to clinical studies. There is hope that these systems can supplement current preclinical drug safety and disposition evaluations, filling gaps and enhancing our ability to predict and understand human relevant toxicities. The International Consortium for Innovation and Quality in Pharmaceutical Development (IQ) MPS Affiliate is a group of pharmaceutical industry scientists who seek to expedite appropriate characterization and incorporation of MPS to potentially improve drug safety assessment and provide safer and more effective medicines to patients. In keeping with this mission, the IQ MPS Affiliate scientists have prepared a series of organotypic manuscripts for several key drug safety and disposition target tissues (lung, liver, kidney, skin, gastrointestinal, cardiovascular, and blood brain barrier/central nervous system). The goal of these manuscripts is to provide key information related to likely initial contexts of use (CoU) and key characterization data needed for incorporation of MPS in pharmaceutical safety screening including a list of characteristic functions, cell types, toxicities, and test agents (representing major mechanisms of toxicity) that can be used by MPS developers. Additional manuscripts focusing on testing biologically based therapeutics and ADME considerations have been prepared as part of this effort. These manuscripts focus on general needs for assessing biologics and ADME endpoints and include similar information to the tissue specific manuscripts where appropriate. The current manuscript is an introduction to several general concepts related to pharmaceutical industry needs with regard to MPS application and other MPS concepts that apply across the organ specific manuscripts.
中文翻译:

介绍在药物安全性和ADME应用中表征和使用微生理系统(MPS)的手稿系列。
尽管构成典型的安全筛选漏斗的众多复杂而漫长的体外测定和体内研究,与安全相关的药物失败仍然是制药公司面临的挑战。缺乏将动物数据完整翻译为人类的原因可以解释其中的一些不足。动物和人类之间在敏感性和药物处置方面的差异也可能起作用。无法潜在地在体外建模的药物潜在靶组织存在许多空白。微生理系统(MPS)可能有助于更好地对这些目标组织进行建模,并提供机会在临床研究之前更好地评估人体安全性的某些方面。希望这些系统可以补充当前的临床前药物安全性和处置评估,填补空白并增强我们预测和理解人类相关毒性的能力。国际药品开发创新与质量协会(IQ)MPS会员协会是一组制药业科学家,他们试图加快MPS的适当表征和纳入,以潜在地改善药物安全性评估并向患者提供更安全,更有效的药物。为了履行这一使命,IQ MPS会员科学家为一系列关键的药物安全性和处置目标组织(肺,肝,肾,皮肤,胃肠道,心血管和血脑屏障/中枢神经系统)准备了一系列器官典型手稿。这些手稿的目的是提供与可能的初始使用环境(CoU)相关的关键信息以及将MPS纳入药物安全性筛查所需的关键特征数据,包括特征功能,细胞类型,毒性和测试试剂的列表(代表MPS开发人员可以使用的主要毒性机制)。作为这项工作的一部分,还准备了其他重点研究基于生物学的疗法和ADME考虑因素的手稿。这些手稿侧重于评估生物制品和ADME终点的一般需求,并在适当的情况下包括与组织特定手稿相似的信息。
更新日期:2020-03-19
中文翻译:

介绍在药物安全性和ADME应用中表征和使用微生理系统(MPS)的手稿系列。
尽管构成典型的安全筛选漏斗的众多复杂而漫长的体外测定和体内研究,与安全相关的药物失败仍然是制药公司面临的挑战。缺乏将动物数据完整翻译为人类的原因可以解释其中的一些不足。动物和人类之间在敏感性和药物处置方面的差异也可能起作用。无法潜在地在体外建模的药物潜在靶组织存在许多空白。微生理系统(MPS)可能有助于更好地对这些目标组织进行建模,并提供机会在临床研究之前更好地评估人体安全性的某些方面。希望这些系统可以补充当前的临床前药物安全性和处置评估,填补空白并增强我们预测和理解人类相关毒性的能力。国际药品开发创新与质量协会(IQ)MPS会员协会是一组制药业科学家,他们试图加快MPS的适当表征和纳入,以潜在地改善药物安全性评估并向患者提供更安全,更有效的药物。为了履行这一使命,IQ MPS会员科学家为一系列关键的药物安全性和处置目标组织(肺,肝,肾,皮肤,胃肠道,心血管和血脑屏障/中枢神经系统)准备了一系列器官典型手稿。这些手稿的目的是提供与可能的初始使用环境(CoU)相关的关键信息以及将MPS纳入药物安全性筛查所需的关键特征数据,包括特征功能,细胞类型,毒性和测试试剂的列表(代表MPS开发人员可以使用的主要毒性机制)。作为这项工作的一部分,还准备了其他重点研究基于生物学的疗法和ADME考虑因素的手稿。这些手稿侧重于评估生物制品和ADME终点的一般需求,并在适当的情况下包括与组织特定手稿相似的信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号