当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the carbon balance for CO2 electroreduction on Cu using gas diffusion electrode reactor designs
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2020/02/12 , DOI: 10.1039/d0ee00047g Ming Ma 1, 2, 3, 4, 5 , Ezra L. Clark 1, 2, 3, 4, 5 , Kasper T. Therkildsen 4, 5, 6, 7, 8 , Sebastian Dalsgaard 1, 2, 3, 4, 5 , Ib Chorkendorff 1, 2, 3, 4, 5 , Brian Seger 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2020/02/12 , DOI: 10.1039/d0ee00047g Ming Ma 1, 2, 3, 4, 5 , Ezra L. Clark 1, 2, 3, 4, 5 , Kasper T. Therkildsen 4, 5, 6, 7, 8 , Sebastian Dalsgaard 1, 2, 3, 4, 5 , Ib Chorkendorff 1, 2, 3, 4, 5 , Brian Seger 1, 2, 3, 4, 5
Affiliation
|
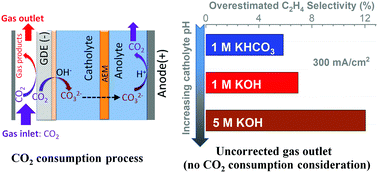
In this work, the carbon balance during high-rate CO2 reduction in flow electrolyzers was rigorously analyzed. The CO2 consumption at gas-diffusion electrodes due to electrochemical conversion and reaction with OH− at the electrode/electrolyte interface leads to a substantial reduction in the volumetric flowrate of gas flow out of the electrolyzer, especially when highly concentrated alkaline electrolytes and elevated current densities are utilized, which is primarily due to an elevated pH at cathode/electrolyte interface. Without considering the CO2 consumption, the faradaic efficiencies for major gas products could be significantly overestimated during high current density CO2 reduction conditions, particularly in the case of high pH electrolyte. In addition, a detailed carbon balance path is elucidated via a two-step procedure of CO2 reaction with OH− at the cathode/electrolyte interface and subsequent CO2 generation at the anode/electrolyte interface caused by a relatively low pH in the vicinity of the anode. Based on the proposed two-step carbon balance path, a systematic exploration of gases released in the anolyte reveals the transformation of a HCO3− or OH− catholyte to a CO32− catholyte, which was further confirmed by pH measurements.
中文翻译:

使用气体扩散电极反应器设计洞悉Cu上电解还原CO2的碳平衡
在这项工作中,严格分析了流动电解槽中高速率CO 2还原过程中的碳平衡。的CO 2消耗的气体扩散电极由于电化学转化和用OH反应-在电极/电解质界面导致在气体的体积流率的显着降低流动电解槽出来,特别是当高浓度碱性电解质和升高的电流利用密度,这主要是由于阴极/电解质界面处的pH升高。如果不考虑CO 2的消耗,在高电流密度的CO 2中,主要气体产品的法拉第效率可能会被高估。还原条件,特别是在高pH电解液中。另外,详细的碳平衡路径阐明经由CO的两步程序2与OH反应-在阴极/电解质界面和随后的CO 2生成在附近引起的相对低的pH的阳极/电解质界面阳极。基于所提出的两步碳平衡的道路上,在阳极液中释放的气体的系统的探索揭示了一个HCO改造3 -或OH -阴极电解液的CO 3 2-的阴极电解液,其进一步通过pH测量的证实。
更新日期:2020-03-19
中文翻译:

使用气体扩散电极反应器设计洞悉Cu上电解还原CO2的碳平衡
在这项工作中,严格分析了流动电解槽中高速率CO 2还原过程中的碳平衡。的CO 2消耗的气体扩散电极由于电化学转化和用OH反应-在电极/电解质界面导致在气体的体积流率的显着降低流动电解槽出来,特别是当高浓度碱性电解质和升高的电流利用密度,这主要是由于阴极/电解质界面处的pH升高。如果不考虑CO 2的消耗,在高电流密度的CO 2中,主要气体产品的法拉第效率可能会被高估。还原条件,特别是在高pH电解液中。另外,详细的碳平衡路径阐明经由CO的两步程序2与OH反应-在阴极/电解质界面和随后的CO 2生成在附近引起的相对低的pH的阳极/电解质界面阳极。基于所提出的两步碳平衡的道路上,在阳极液中释放的气体的系统的探索揭示了一个HCO改造3 -或OH -阴极电解液的CO 3 2-的阴极电解液,其进一步通过pH测量的证实。











































 京公网安备 11010802027423号
京公网安备 11010802027423号