当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical studies on the N–X (X = Cl, O) bond activation mechanism in catalytic C–H amination
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020/02/11 , DOI: 10.1039/c9cy02555c Yang Yu 1, 2, 3, 4, 5 , Gen Luo 5, 6, 7, 8 , Jimin Yang 1, 2, 3, 4, 5 , Yi Luo 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020/02/11 , DOI: 10.1039/c9cy02555c Yang Yu 1, 2, 3, 4, 5 , Gen Luo 5, 6, 7, 8 , Jimin Yang 1, 2, 3, 4, 5 , Yi Luo 1, 2, 3, 4, 5
Affiliation
|
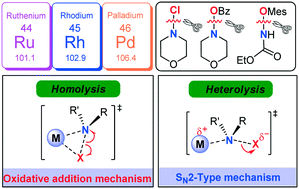
A comprehensive DFT study has been performed on the mechanism of Rh-catalysed C–H amination of N-arylbenzamide with N-chloromorpholine. The catalytic cycle starting from the N–H deprotonation of N-arylbenzamide was ruled out due to the inconsistency between the calculated and experimentally observed rate-determining steps. Instead, the CsOAc induced pre-deprotonated form of N-arylbenzamide participates in the catalytic cycle. The reaction then goes through C–H activation via concerted metalation–deprotonation, N–Cl cleavage, reductive elimination, and protonation to complete the catalytic cycle. It has been found that an SN2-type N–Cl cleavage fashion is more favorable compared with the conventional oxidative addition manner. Both cleavage manners of the N–Cl bond were manifested by geometry structure and orbital population analyses. More importantly, such an SN2-type mechanism also works for Ru-catalysed N–Cl cleavage and Rh-/Ru-/Pd-catalysed N–O cleavage involved in C–H amination systems. It is found that the less geometrical distortion accounts for the preference towards the SN2-type. It is theoretically demonstrated that the manner of N–O cleavage is affected by the ligand substituents. Modification of N-benzoyloxymorpholine with an electron-withdrawing substituent could facilitate the SN2-type N–O cleavage process. Such an SN2-type N–X (X = N, O) cleavage process was established for the first time in the field of C–H amination, which enriches the C–H amination chemistry.
中文翻译:

N–X(X = Cl,O)键在催化CH–H胺化中活化机理的理论研究
DFT研究已经进行了全面的DFT研究,研究了R-催化N-芳基苯甲酰胺与N-氯吗啉的C–H胺化。由于计算的和实验观察到的速率决定步骤之间的不一致,排除了从N-芳基苯甲酰胺的NH脱质子化开始的催化循环。相反,CsOAc诱导的N-芳基苯甲酰胺的预去质子化形式参与了催化循环。然后,该反应通过协同的金属化-去质子化,N-Cl裂解,还原消除和质子化作用通过CH活化,从而完成催化循环。已经发现一个S N与传统的氧化加成方式相比,2型N–Cl的裂解方式更为有利。N–Cl键的两种裂解方式均通过几何结构和轨道种群分析得以体现。更重要的是,这种S N 2型机制也可用于参与C–H胺化系统的Ru催化的N–Cl裂解和Rh- / Ru- / Pd催化的N–O裂解。据发现,较少几何畸变占朝向S上的偏好Ñ 2型。从理论上讲,N–O裂解的方式受配体取代基的影响。用吸电子取代基修饰N-苯甲酰氧基吗啉可以促进S N 2型N–O裂解过程。这样的S Ñ2型N–X(X = N,O)裂解过程是在C–H胺化领域中首次建立的,这丰富了C–H胺化的化学过程。
更新日期:2020-03-26
中文翻译:

N–X(X = Cl,O)键在催化CH–H胺化中活化机理的理论研究
DFT研究已经进行了全面的DFT研究,研究了R-催化N-芳基苯甲酰胺与N-氯吗啉的C–H胺化。由于计算的和实验观察到的速率决定步骤之间的不一致,排除了从N-芳基苯甲酰胺的NH脱质子化开始的催化循环。相反,CsOAc诱导的N-芳基苯甲酰胺的预去质子化形式参与了催化循环。然后,该反应通过协同的金属化-去质子化,N-Cl裂解,还原消除和质子化作用通过CH活化,从而完成催化循环。已经发现一个S N与传统的氧化加成方式相比,2型N–Cl的裂解方式更为有利。N–Cl键的两种裂解方式均通过几何结构和轨道种群分析得以体现。更重要的是,这种S N 2型机制也可用于参与C–H胺化系统的Ru催化的N–Cl裂解和Rh- / Ru- / Pd催化的N–O裂解。据发现,较少几何畸变占朝向S上的偏好Ñ 2型。从理论上讲,N–O裂解的方式受配体取代基的影响。用吸电子取代基修饰N-苯甲酰氧基吗啉可以促进S N 2型N–O裂解过程。这样的S Ñ2型N–X(X = N,O)裂解过程是在C–H胺化领域中首次建立的,这丰富了C–H胺化的化学过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号