当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering an alcohol dehydrogenase with enhanced activity and stereoselectivity toward diaryl ketones: reduction of steric hindrance and change of the stereocontrol element
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020/01/21 , DOI: 10.1039/c9cy02444a Kai Wu 1, 2, 3, 4, 5 , Zhijun Yang 1, 2, 3, 4, 5 , Xiangguo Meng 1, 2, 3, 4, 5 , Rong Chen 1, 2, 3, 4 , Jiankun Huang 1, 2, 3, 4 , Lei Shao 2, 3, 4, 5, 6
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020/01/21 , DOI: 10.1039/c9cy02444a Kai Wu 1, 2, 3, 4, 5 , Zhijun Yang 1, 2, 3, 4, 5 , Xiangguo Meng 1, 2, 3, 4, 5 , Rong Chen 1, 2, 3, 4 , Jiankun Huang 1, 2, 3, 4 , Lei Shao 2, 3, 4, 5, 6
Affiliation
|
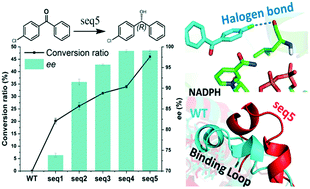
Steric hindrance in the binding pocket of an alcohol dehydrogenase (ADH) has a great impact on its activity and stereoselectivity simultaneously. Due to the subtle structural difference between two bulky phenyl substituents, the asymmetric synthesis of diaryl alcohols by bioreduction of diaryl ketones is often hindered by the low activity and stereoselectivity of ADHs. To engineer an ADH with practical properties and to investigate the molecular mechanism behind the asymmetric biocatalysis of diaryl ketones, we engineered an ADH from Lactobacillus kefiri (LkADH) to asymmetrically catalyse the reduction of 4-chlorodiphenylketones (CPPK), which are not catalysed by the wild type (WT) enzyme. Mutants seq1–seq5 with gradually increased activity and stereoselectivity were obtained through iterative “shrinking mutagenesis.” The final mutant seq5 (Y190P/I144V/L199V/E145C/M206F) demonstrated the highest activity and excellent stereoselectivity of >99% ee. Molecular simulation analyses revealed that mutations may enhance the activity by eliminating steric hindrance, inducing a more open binding loop and constructing more noncovalent interactions. The pro-R pose of CPPK with a halogen bond formed a pre-reaction conformation more easily than the pro-S pose, resulting in the high ee of (R)-CPPO in seq5. Moreover, different halogen bonds formed due to the different positions of chlorine substituents, resulting in opposite substrate binding orientation and stereoselectivity. Therefore, the stereoselectivity of seq5 was inverted toward ortho- rather than para-chlorine substituted ketones. These results indicate that the stereocontrol element of LkADH was changed to recognise diaryl ketones after steric hindrance was eliminated. This study provides novel insights into the role of steric hindrance and noncovalent bonds in the determination of the activity and stereoselectivity of enzymes, and presents an approach producing key intermediates of chiral drugs with practical potential.
中文翻译:

工程设计具有增强的活性和对二芳基酮的立体选择性的醇脱氢酶:减少空间位阻和改变立体控制元件
醇脱氢酶(ADH)的结合口袋中的位阻同时对其活性和立体选择性产生很大影响。由于两个庞大的苯基取代基之间的微妙结构差异,ADH的低活性和立体选择性常常阻碍了通过生物还原二芳基酮进行不对称合成二芳基醇。为了设计具有实用性能的ADH并研究二芳基酮的不对称生物催化背后的分子机理,我们设计了开菲乳杆菌的ADH。(LkADH)可不对称地催化野生型(WT)酶未催化的4-氯二苯甲酮(CPPK)的还原。通过反复的“收缩诱变”获得了活性和立体选择性逐渐提高的突变体seq1-seq5。最终的突变体seq5(Y190P / I144V / L199V / E145C / M206F)表现出最高的活性和出色的立体选择性(> 99%ee)。分子模拟分析表明,突变可通过消除空间位阻,诱导更开放的结合环并构建更多非共价相互作用来增强活性。亲ř与卤素键CPPK的姿态形成的预反应的构象更容易地比亲小号姿态,导致高ee值的(seq5中的R)-CPPO。此外,由于氯取代基的位置不同,形成了不同的卤素键,导致相反的底物结合方向和立体选择性。因此,seq5的立体选择性向邻位取代而不是对氯取代。这些结果表明,消除空间位阻后,LkADH的立体控制元件被改变为识别二芳基酮。这项研究提供了对空间位阻和非共价键在确定酶的活性和立体选择性中的作用的新颖见解,并提出了一种生产具有实际潜力的手性药物关键中间体的方法。
更新日期:2020-03-26
中文翻译:

工程设计具有增强的活性和对二芳基酮的立体选择性的醇脱氢酶:减少空间位阻和改变立体控制元件
醇脱氢酶(ADH)的结合口袋中的位阻同时对其活性和立体选择性产生很大影响。由于两个庞大的苯基取代基之间的微妙结构差异,ADH的低活性和立体选择性常常阻碍了通过生物还原二芳基酮进行不对称合成二芳基醇。为了设计具有实用性能的ADH并研究二芳基酮的不对称生物催化背后的分子机理,我们设计了开菲乳杆菌的ADH。(LkADH)可不对称地催化野生型(WT)酶未催化的4-氯二苯甲酮(CPPK)的还原。通过反复的“收缩诱变”获得了活性和立体选择性逐渐提高的突变体seq1-seq5。最终的突变体seq5(Y190P / I144V / L199V / E145C / M206F)表现出最高的活性和出色的立体选择性(> 99%ee)。分子模拟分析表明,突变可通过消除空间位阻,诱导更开放的结合环并构建更多非共价相互作用来增强活性。亲ř与卤素键CPPK的姿态形成的预反应的构象更容易地比亲小号姿态,导致高ee值的(seq5中的R)-CPPO。此外,由于氯取代基的位置不同,形成了不同的卤素键,导致相反的底物结合方向和立体选择性。因此,seq5的立体选择性向邻位取代而不是对氯取代。这些结果表明,消除空间位阻后,LkADH的立体控制元件被改变为识别二芳基酮。这项研究提供了对空间位阻和非共价键在确定酶的活性和立体选择性中的作用的新颖见解,并提出了一种生产具有实际潜力的手性药物关键中间体的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号