当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rh‐Catalyzed Reaction of Propargylic Alcohols with Aryl Boronic Acids–Switch from β‐OH Elimination to Protodemetalation
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-03-05 , DOI: 10.1002/cjoc.202000044 Weiyi Wang 1 , Hui Qian 1 , Shengming Ma 1, 2
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-03-05 , DOI: 10.1002/cjoc.202000044 Weiyi Wang 1 , Hui Qian 1 , Shengming Ma 1, 2
Affiliation
|
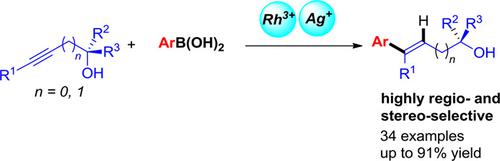
It is known that Rh‐catalyzed reaction of propargylic alcohols with aryl metallic reagents undergoes SN2’‐type reaction affording allenes via a sequential arylmetalation and β‐OH elimination process. Here we report a Rh/Ag‐cocatalyzed reaction of propargylic alcohols with organoboronic acids affording stereo‐defined (E)‐3‐arylallylic alcohols via arylmetalation and protodemetalation with a high regio‐ and stereoselectivity under very mild conditions. The reaction exhibits a good substrate scope and the compatibility with synthetically useful functional groups with no racemization for optically active propargylic alcohols. Such a reaction may also be extended to homopropargylic alcohols with a remarkable regioselectivity and exclusive E‐stereoselectivity.
中文翻译:

丙醇与芳基硼酸的Rh催化反应-从消除β-OH到原金属脱金属
众所周知,炔丙醇与芳基金属试剂的Rh催化反应会经历S N 2'型反应,这是通过顺序的芳基金属化和β-OH消除过程提供的。在此我们报道了炔丙醇与有机硼酸的Rh / Ag催化反应,在非常温和的条件下,通过芳基金属化和原金属脱金属以高区域和立体选择性提供了立体定义的(E)-3-芳基烯丙醇。该反应显示出良好的底物范围以及与合成有用的官能团的相容性,而对于旋光的炔丙醇没有外消旋作用。这种反应还可以扩展为具有显着的区域选择性和排他性E-立体选择性的均丙醇。
更新日期:2020-03-05
中文翻译:

丙醇与芳基硼酸的Rh催化反应-从消除β-OH到原金属脱金属
众所周知,炔丙醇与芳基金属试剂的Rh催化反应会经历S N 2'型反应,这是通过顺序的芳基金属化和β-OH消除过程提供的。在此我们报道了炔丙醇与有机硼酸的Rh / Ag催化反应,在非常温和的条件下,通过芳基金属化和原金属脱金属以高区域和立体选择性提供了立体定义的(E)-3-芳基烯丙醇。该反应显示出良好的底物范围以及与合成有用的官能团的相容性,而对于旋光的炔丙醇没有外消旋作用。这种反应还可以扩展为具有显着的区域选择性和排他性E-立体选择性的均丙醇。











































 京公网安备 11010802027423号
京公网安备 11010802027423号