当前位置:
X-MOL 学术
›
Lancet Neurol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Advances in oral immunomodulating therapies in relapsing multiple sclerosis
The Lancet Neurology ( IF 46.5 ) Pub Date : 2020-04-01 , DOI: 10.1016/s1474-4422(19)30391-6 Tobias Derfuss 1 , Matthias Mehling 1 , Athina Papadopoulou 2 , Amit Bar-Or 3 , Jeffrey A Cohen 4 , Ludwig Kappos 5
The Lancet Neurology ( IF 46.5 ) Pub Date : 2020-04-01 , DOI: 10.1016/s1474-4422(19)30391-6 Tobias Derfuss 1 , Matthias Mehling 1 , Athina Papadopoulou 2 , Amit Bar-Or 3 , Jeffrey A Cohen 4 , Ludwig Kappos 5
Affiliation
|
BACKGROUND
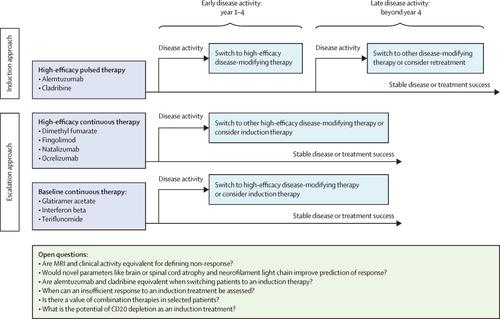
Oral treatment options for disease-modifying therapy in relapsing multiple sclerosis have substantially increased over the past decade with four approved oral compounds now available: fingolimod, dimethyl fumarate, teriflunomide, and cladribine. Although these immunomodulating therapies are all orally administered, and thus convenient for patients, they have different modes of action. These distinct mechanisms of action allow better adaption of treatments according to individual comorbidities and offer different mechanisms of treatment such as inhibition of immune cell trafficking versus immune cell depletion, thereby substantially expanding the available treatment options. RECENT DEVELOPMENTS
New sphingosine-1-phosphate receptor (S1PR) modulators with more specific S1PR target profiles and potentially better safety profiles compared with fingolimod were tested in patients with relapsing multiple sclerosis. For example, siponimod, which targets S1PR1 and S1PR5, was approved in March, 2019, by the US Food and Drug Administration for the treatment of relapsing multiple sclerosis including active secondary progressive multiple sclerosis. Ozanimod, another S1P receptor modulator in the approval stage that also targets S1PR1 and S1PR5, reduced relapse rates and MRI activity in two phase 3 trials of patients with relapsing multiple sclerosis. Blocking of matrix metalloproteinases or tyrosine kinases are novel modes of action in the treatment of relapsing multiple sclerosis, which are exhibited by minocycline and evobrutinib, respectively. Minocycline reduced conversion to multiple sclerosis in patients with a clinically isolated syndrome. Evobrutinib reduced MRI activity in a phase 2 trial, and a phase 3 trial is underway, in patients with relapsing multiple sclerosis. Diroximel fumarate is metabolised to monomethyl fumarate, the active metabolite of dimethyl fumarate, reduces circulating lymphocytes and modifies the activation profile of monocytes, and is being tested in this disease with the aim to improve gastrointestinal tolerability. The oral immunomodulator laquinimod did not reach the primary endpoint of reduction in confirmed disability progression in a phase 3 trial of patients with relapsing multiple sclerosis. In a phase 2 trial of patients with primary progressive multiple sclerosis, laquinimod also did not reach the primary endpoint of a reduction in brain volume loss, as a consequence the development of this drug will probably not be continued in multiple sclerosis. WHERE NEXT?: Several new oral compounds are in late-stage clinical development. With new modes of action introduced to the treatment of multiple sclerosis, the question of how to select and sequence different treatments in individual patients arises. Balancing risks with the expected efficacy of disease-modifying therapies will still be key for treatment selection. However, risks as well as efficacy can change when moving from the controlled clinical trial setting to clinical practice. Because some oral treatments, such as cladribine, have long-lasting effects on the immune system, the cumulative effects of sequential monotherapies can resemble the effects of a concurrent combination therapy. This treatment scheme might lead to higher efficacy but also to new safety concerns. These sequential treatments were largely excluded in phase 2 and 3 trials; therefore, monitoring both short-term and long-term effects of sequential disease-modifying therapies in phase 4 studies, cohort studies, and registries will be necessary.
中文翻译:

复发性多发性硬化的口服免疫调节疗法进展
背景 复发性多发性硬化症的疾病缓解疗法的口服治疗选择在过去十年中显着增加,现已有四种获批的口服化合物可用:芬戈莫德、富马酸二甲酯、特立氟胺和克拉屈滨。虽然这些免疫调节疗法都是口服给药,方便患者,但它们的作用方式不同。这些不同的作用机制允许根据个体合并症更好地适应治疗,并提供不同的治疗机制,例如抑制免疫细胞运输与免疫细胞耗竭,从而大大扩展了可用的治疗选择。最近的发展 在复发性多发性硬化症患者中测试了新的 1-磷酸鞘氨醇受体 (S1PR) 调节剂,与芬戈莫德相比,其具有更具体的 S1PR 目标特征和潜在更好的安全性特征。例如,针对 S1PR1 和 S1PR5 的辛波莫德于 2019 年 3 月被美国食品和药物管理局批准用于治疗复发性多发性硬化症,包括活动性继发性进行性多发性硬化症。Ozanimod 是另一种处于批准阶段的 S1P 受体调节剂,也针对 S1PR1 和 S1PR5,在复发性多发性硬化症患者的两项 3 期试验中降低了复发率和 MRI 活性。基质金属蛋白酶或酪氨酸激酶的阻断是治疗复发性多发性硬化症的新作用模式,分别由米诺环素和依布替尼表现出。米诺环素减少了临床孤立综合征患者向多发性硬化症的转化。Evobrutinib 在一项 2 期试验中降低了 MRI 活性,并且一项 3 期试验正在进行中,用于复发性多发性硬化症患者。Diroximel fumarate 被代谢为富马酸单甲酯,即富马酸二甲酯的活性代谢物,减少循环淋巴细胞并改变单核细胞的激活谱,并正在该疾病中进行测试,目的是提高胃肠道耐受性。在复发性多发性硬化症患者的 3 期试验中,口服免疫调节剂拉喹莫德没有达到减少已确认的残疾进展的主要终点。在原发性进行性多发性硬化症患者的 2 期试验中,拉喹莫德也没有达到减少脑容量损失的主要终点,因此这种药物的开发可能不会继续用于治疗多发性硬化症。下一步在哪里?:几种新的口服化合物正处于后期临床开发阶段。随着治疗多发性硬化症的新作用模式的引入,出现了如何在个体患者中选择和排序不同治疗的问题。平衡风险与疾病修饰疗法的预期疗效仍将是治疗选择的关键。然而,当从受控临床试验环境转移到临床实践时,风险和疗效可能会发生变化。因为一些口服治疗,如克拉屈滨,对免疫系统有长期影响,序贯单一疗法的累积效应类似于同步联合疗法的效应。这种治疗方案可能会带来更高的疗效,但也会带来新的安全问题。2 期和 3 期试验基本上排除了这些序贯治疗;因此,有必要在 4 期研究、队列研究和登记中监测序贯疾病改善疗法的短期和长期效果。
更新日期:2020-04-01
中文翻译:

复发性多发性硬化的口服免疫调节疗法进展
背景 复发性多发性硬化症的疾病缓解疗法的口服治疗选择在过去十年中显着增加,现已有四种获批的口服化合物可用:芬戈莫德、富马酸二甲酯、特立氟胺和克拉屈滨。虽然这些免疫调节疗法都是口服给药,方便患者,但它们的作用方式不同。这些不同的作用机制允许根据个体合并症更好地适应治疗,并提供不同的治疗机制,例如抑制免疫细胞运输与免疫细胞耗竭,从而大大扩展了可用的治疗选择。最近的发展 在复发性多发性硬化症患者中测试了新的 1-磷酸鞘氨醇受体 (S1PR) 调节剂,与芬戈莫德相比,其具有更具体的 S1PR 目标特征和潜在更好的安全性特征。例如,针对 S1PR1 和 S1PR5 的辛波莫德于 2019 年 3 月被美国食品和药物管理局批准用于治疗复发性多发性硬化症,包括活动性继发性进行性多发性硬化症。Ozanimod 是另一种处于批准阶段的 S1P 受体调节剂,也针对 S1PR1 和 S1PR5,在复发性多发性硬化症患者的两项 3 期试验中降低了复发率和 MRI 活性。基质金属蛋白酶或酪氨酸激酶的阻断是治疗复发性多发性硬化症的新作用模式,分别由米诺环素和依布替尼表现出。米诺环素减少了临床孤立综合征患者向多发性硬化症的转化。Evobrutinib 在一项 2 期试验中降低了 MRI 活性,并且一项 3 期试验正在进行中,用于复发性多发性硬化症患者。Diroximel fumarate 被代谢为富马酸单甲酯,即富马酸二甲酯的活性代谢物,减少循环淋巴细胞并改变单核细胞的激活谱,并正在该疾病中进行测试,目的是提高胃肠道耐受性。在复发性多发性硬化症患者的 3 期试验中,口服免疫调节剂拉喹莫德没有达到减少已确认的残疾进展的主要终点。在原发性进行性多发性硬化症患者的 2 期试验中,拉喹莫德也没有达到减少脑容量损失的主要终点,因此这种药物的开发可能不会继续用于治疗多发性硬化症。下一步在哪里?:几种新的口服化合物正处于后期临床开发阶段。随着治疗多发性硬化症的新作用模式的引入,出现了如何在个体患者中选择和排序不同治疗的问题。平衡风险与疾病修饰疗法的预期疗效仍将是治疗选择的关键。然而,当从受控临床试验环境转移到临床实践时,风险和疗效可能会发生变化。因为一些口服治疗,如克拉屈滨,对免疫系统有长期影响,序贯单一疗法的累积效应类似于同步联合疗法的效应。这种治疗方案可能会带来更高的疗效,但也会带来新的安全问题。2 期和 3 期试验基本上排除了这些序贯治疗;因此,有必要在 4 期研究、队列研究和登记中监测序贯疾病改善疗法的短期和长期效果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号