当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
α-Ketoamides as Broad-Spectrum Inhibitors of Coronavirus and Enterovirus Replication: Structure-Based Design, Synthesis, and Activity Assessment.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-02-24 , DOI: 10.1021/acs.jmedchem.9b01828 Linlin Zhang 1, 2 , Daizong Lin 1, 2, 3 , Yuri Kusov 1 , Yong Nian 3 , Qingjun Ma 1 , Jiang Wang 3 , Albrecht von Brunn 4 , Pieter Leyssen 5 , Kristina Lanko 5 , Johan Neyts 5 , Adriaan de Wilde 6 , Eric J Snijder 6 , Hong Liu 3 , Rolf Hilgenfeld 1, 2, 3
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-02-24 , DOI: 10.1021/acs.jmedchem.9b01828 Linlin Zhang 1, 2 , Daizong Lin 1, 2, 3 , Yuri Kusov 1 , Yong Nian 3 , Qingjun Ma 1 , Jiang Wang 3 , Albrecht von Brunn 4 , Pieter Leyssen 5 , Kristina Lanko 5 , Johan Neyts 5 , Adriaan de Wilde 6 , Eric J Snijder 6 , Hong Liu 3 , Rolf Hilgenfeld 1, 2, 3
Affiliation
|
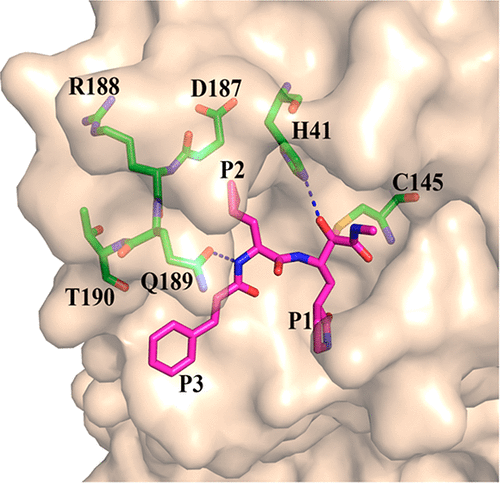
The main protease of coronaviruses and the 3C protease of enteroviruses share a similar active-site architecture and a unique requirement for glutamine in the P1 position of the substrate. Because of their unique specificity and essential role in viral polyprotein processing, these proteases are suitable targets for the development of antiviral drugs. In order to obtain near-equipotent, broad-spectrum antivirals against alphacoronaviruses, betacoronaviruses, and enteroviruses, we pursued a structure-based design of peptidomimetic α-ketoamides as inhibitors of main and 3C proteases. Six crystal structures of protease-inhibitor complexes were determined as part of this study. Compounds synthesized were tested against the recombinant proteases as well as in viral replicons and virus-infected cell cultures; most of them were not cell-toxic. Optimization of the P2 substituent of the α-ketoamides proved crucial for achieving near-equipotency against the three virus genera. The best near-equipotent inhibitors, 11u (P2 = cyclopentylmethyl) and 11r (P2 = cyclohexylmethyl), display low-micromolar EC50 values against enteroviruses, alphacoronaviruses, and betacoronaviruses in cell cultures. In Huh7 cells, 11r exhibits three-digit picomolar activity against the Middle East Respiratory Syndrome coronavirus.
中文翻译:

α-酮酰胺作为冠状病毒和肠道病毒复制的广谱抑制剂:基于结构的设计、合成和活性评估。
冠状病毒的主要蛋白酶和肠道病毒的3C蛋白酶具有相似的活性位点结构,并且对底物P1位置的谷氨酰胺有独特的需求。由于其独特的特异性和在病毒多蛋白加工中的重要作用,这些蛋白酶是开发抗病毒药物的合适靶标。为了获得针对α冠状病毒、β冠状病毒和肠道病毒的近乎等效的广谱抗病毒药物,我们对拟肽α-酮酰胺进行了基于结构的设计,作为主要蛋白酶和3C蛋白酶的抑制剂。作为本研究的一部分,确定了蛋白酶抑制剂复合物的六种晶体结构。合成的化合物针对重组蛋白酶以及病毒复制子和病毒感染的细胞培养物进行了测试;其中大多数没有细胞毒性。 α-酮酰胺的 P2 取代基的优化被证明对于实现针对三种病毒属的近等效性至关重要。最好的近等效抑制剂 11u(P2 = 环戊基甲基)和 11r(P2 = 环己基甲基)在细胞培养物中针对肠道病毒、α 冠状病毒和 β 冠状病毒表现出低微摩尔 EC50 值。在 Huh7 细胞中,11r 对中东呼吸综合征冠状病毒表现出三位数皮摩尔活性。
更新日期:2020-02-11
中文翻译:

α-酮酰胺作为冠状病毒和肠道病毒复制的广谱抑制剂:基于结构的设计、合成和活性评估。
冠状病毒的主要蛋白酶和肠道病毒的3C蛋白酶具有相似的活性位点结构,并且对底物P1位置的谷氨酰胺有独特的需求。由于其独特的特异性和在病毒多蛋白加工中的重要作用,这些蛋白酶是开发抗病毒药物的合适靶标。为了获得针对α冠状病毒、β冠状病毒和肠道病毒的近乎等效的广谱抗病毒药物,我们对拟肽α-酮酰胺进行了基于结构的设计,作为主要蛋白酶和3C蛋白酶的抑制剂。作为本研究的一部分,确定了蛋白酶抑制剂复合物的六种晶体结构。合成的化合物针对重组蛋白酶以及病毒复制子和病毒感染的细胞培养物进行了测试;其中大多数没有细胞毒性。 α-酮酰胺的 P2 取代基的优化被证明对于实现针对三种病毒属的近等效性至关重要。最好的近等效抑制剂 11u(P2 = 环戊基甲基)和 11r(P2 = 环己基甲基)在细胞培养物中针对肠道病毒、α 冠状病毒和 β 冠状病毒表现出低微摩尔 EC50 值。在 Huh7 细胞中,11r 对中东呼吸综合征冠状病毒表现出三位数皮摩尔活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号