Electrochemistry Communications ( IF 4.7 ) Pub Date : 2020-02-12 , DOI: 10.1016/j.elecom.2020.106685 Hailemariam Kassa Bezabh , Meng-Che Tsai , Tesfaye Teka Hagos , Tamene Tadesse Beyene , Gebregziabher Brhane Berhe , Teklay Mezgebe Hagos , Ljalem Hadush Abrha , Shuo-Feng Chiu , Wei-Nien Su , Bing Joe Hwang
|
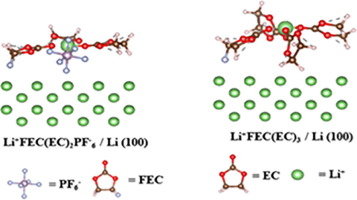
Electrolyte for lithium-ion batteries should facilitate Li+-ion transport and control solid electrolyte interface(SEI). To improve their performance, most of the researches focused more on the effect of the solvation structure of bulk electrolytes on the SEI formation rather than adsorbed species on the Li-anode surface in the past. However, it was found recently that the adsorbed species on the Li-anode surface are strongly connected with the formation of SEI. In this study, diluted [Li+ fluoroethylene carbonate (FEC)/ethylene carbonate(EC)n, Li+(EC)n (n=1-4)] , and concentrated (Li+FEC(EC)n(PF6-) (n=0-3) electrolyte are investigated in bulk phase and on Li-anode surface using density functional theory (DFT). The stability of the clusters is investigated by their solvation energy and Gibbs free energy change. From the Gibbs free energy change of the clusters, it is found that Li+(EC)4 cluster is the most stable structure in the bulk phase of the diluted electrolyte in the presence or absence of FEC. Pure EC solvated Li+-ion species in both dilute and concentrated electrolytes in bulk solution is more stable, the adsorption energy of pure EC-solvated Li+-ion species on Li+-anode surface was found weaker than EC and FEC co-solvated Li+-ion species in the diluted electrolyte. DFT calculation suggesting that the dominant species on the Li-anode surface are found Li+FEC(EC)3 and Li+FEC(EC)2PF-6 in a diluted and concentrated electrolyte, respectively. The decomposition of the anion-rich adsorbed species in the concentrated electrolyte is suggested to form a better SEI to stabilize Li-anode compared to anion-free adsorbed species in a diluted electrolyte.
中文翻译:

成膜添加剂在锂金属电池稀释和浓缩电解质中的作用:基于密度泛函理论的方法
锂离子电池的电解质应促进Li +离子的传输并控制固体电解质界面(SEI)。为了提高其性能,大多数研究更多地集中在本体电解质的溶剂化结构对SEI形成的影响上,而不是过去在锂阳极表面上的吸附物质上。然而,最近发现锂阳极表面上的吸附物质与SEI的形成紧密相关。在这项研究中,稀释[李+氟代碳酸(FEC)/碳酸亚乙酯(EC)N,栗+(EC)N(N = 1-4)],并浓缩(栗+ FEC(EC)Ñ(PF 6 -)使用密度泛函理论(DFT)在体相中和在Li阳极表面上研究了(n = 0-3)电解质。通过其溶剂化能和吉布斯自由能变化研究了团簇的稳定性。从簇的吉布斯自由能变化中,发现在存在或不存在FEC的情况下,Li +(EC)4簇在稀释电解质的本体相中是最稳定的结构。稀溶液和浓电解质中的纯EC溶剂化的Li +离子物质在溶液中都更稳定,发现纯EC溶剂化的Li +离子物质在Li +阳极表面的吸附能弱于EC和FEC共溶剂李+稀释电解液中的离子种类。DFT计算表明,在稀和浓电解质中分别发现了Li阳极表面上的优势物质Li + FEC(EC)3和Li + FEC(EC)2 PF - 6。与稀释电解质中无阴离子吸附物质相比,建议浓缩电解质中富阴离子吸附物质的分解形成更好的SEI,以稳定锂阳极。











































 京公网安备 11010802027423号
京公网安备 11010802027423号