当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A combined periodic DFT and QM/MM approach to understand the radical mechanism of the catalytic production of methanol from glycerol
Faraday Discussions ( IF 3.3 ) Pub Date : 2020-2-12 , DOI: 10.1039/d0fd00005a Mala A Sainna 1 , Sachin Nanavati 1 , Constance Black 1 , Louise Smith 1 , Karl Mugford 1 , Harry Jenkins 1 , Mark Douthwaite 1 , Nicholas F Dummer 1 , C Richard A Catlow 1 , Graham J Hutchings 1 , Stuart H Taylor 1 , Andrew J Logsdail 1 , David J Willock 1
Faraday Discussions ( IF 3.3 ) Pub Date : 2020-2-12 , DOI: 10.1039/d0fd00005a Mala A Sainna 1 , Sachin Nanavati 1 , Constance Black 1 , Louise Smith 1 , Karl Mugford 1 , Harry Jenkins 1 , Mark Douthwaite 1 , Nicholas F Dummer 1 , C Richard A Catlow 1 , Graham J Hutchings 1 , Stuart H Taylor 1 , Andrew J Logsdail 1 , David J Willock 1
Affiliation
|
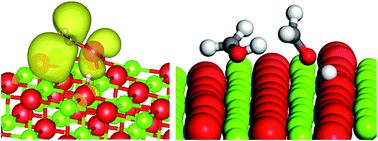
The production of methanol from glycerol over a basic oxide, such as MgO, using high reaction temperatures (320 °C) is a promising new approach to improving atom efficiency in the production of biofuels. The mechanism of this reaction involves the homolytic cleavage of the C3 feedstock, or its dehydration product hydroxyacetone, to produce a hydroxymethyl radical species which can then abstract an H atom from other species. Obtaining a detailed reaction mechanism for this type of chemistry is difficult due to the large number of products present when the system is operated at high conversions. In this contribution we show how DFT based modelling studies can provide new insights into likely reaction pathways, in particular the source of H atoms for the final step of converting hydroxymethyl radicals to methanol. We show that water is unlikely to be important in this stage of the process, C–H bonds of C2 and C3 species can give an energetically favourable pathway and that the disproportionation of hydroxymethyl radicals to methanol and formaldehyde produces a very favourable route. Experimental analysis of reaction products confirms the presence of formaldehyde. The calculations presented in this work also provide new insight into the role of the catalyst surface in the reaction showing that the base sites of the MgO(100) are able to deprotonate hydroxymethyl radicals but not methanol itself. In carrying out the calculations we also show how periodic DFT and QM/MM approaches can be used together to obtain a rounded picture of molecular adsorption to surfaces and homolytic bond cleavage which are both central to the reactions studied.
中文翻译:

结合周期性 DFT 和 QM/MM 方法来了解从甘油催化生产甲醇的自由基机制
使用高反应温度 (320 °C) 从甘油在碱性氧化物(如 MgO)上生产甲醇是提高生物燃料生产中原子效率的一种有前途的新方法。该反应的机制涉及 C 3的均裂原料或其脱水产物羟基丙酮,以产生羟甲基自由基物种,然后可以从其他物种中提取 H 原子。由于系统在高转化率下运行时存在大量产物,因此很难获得此类化学的详细反应机理。在这篇文章中,我们展示了基于 DFT 的建模研究如何为可能的反应途径提供新的见解,特别是将羟甲基自由基转化为甲醇的最后一步的 H 原子来源。我们表明水在这个过程的这个阶段不太可能是重要的,C 2和 C 3 的C-H 键物种可以提供能量上有利的途径,并且羟甲基自由基歧化为甲醇和甲醛会产生非常有利的途径。反应产物的实验分析证实了甲醛的存在。这项工作中提出的计算还提供了对催化剂表面在反应中的作用的新见解,表明 MgO(100) 的碱位能够使羟甲基自由基去质子化,但不能使甲醇本身去质子化。在进行计算时,我们还展示了如何将周期性 DFT 和 QM/MM 方法一起使用以获得分子吸附到表面和均裂键裂解的圆形图,这两个都是研究反应的核心。
更新日期:2020-02-12
中文翻译:

结合周期性 DFT 和 QM/MM 方法来了解从甘油催化生产甲醇的自由基机制
使用高反应温度 (320 °C) 从甘油在碱性氧化物(如 MgO)上生产甲醇是提高生物燃料生产中原子效率的一种有前途的新方法。该反应的机制涉及 C 3的均裂原料或其脱水产物羟基丙酮,以产生羟甲基自由基物种,然后可以从其他物种中提取 H 原子。由于系统在高转化率下运行时存在大量产物,因此很难获得此类化学的详细反应机理。在这篇文章中,我们展示了基于 DFT 的建模研究如何为可能的反应途径提供新的见解,特别是将羟甲基自由基转化为甲醇的最后一步的 H 原子来源。我们表明水在这个过程的这个阶段不太可能是重要的,C 2和 C 3 的C-H 键物种可以提供能量上有利的途径,并且羟甲基自由基歧化为甲醇和甲醛会产生非常有利的途径。反应产物的实验分析证实了甲醛的存在。这项工作中提出的计算还提供了对催化剂表面在反应中的作用的新见解,表明 MgO(100) 的碱位能够使羟甲基自由基去质子化,但不能使甲醇本身去质子化。在进行计算时,我们还展示了如何将周期性 DFT 和 QM/MM 方法一起使用以获得分子吸附到表面和均裂键裂解的圆形图,这两个都是研究反应的核心。









































 京公网安备 11010802027423号
京公网安备 11010802027423号