当前位置:
X-MOL 学术
›
Neuropharmacology
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chronic alcohol disrupts hypothalamic responses to stress by modifying CRF and NMDA receptor function.
Neuropharmacology ( IF 4.7 ) Pub Date : 2020-02-12 , DOI: 10.1016/j.neuropharm.2020.107991 Vincent N Marty 1 , Yatendra Mulpuri 1 , Joseph J Munier 1 , Igor Spigelman 2
Neuropharmacology ( IF 4.7 ) Pub Date : 2020-02-12 , DOI: 10.1016/j.neuropharm.2020.107991 Vincent N Marty 1 , Yatendra Mulpuri 1 , Joseph J Munier 1 , Igor Spigelman 2
Affiliation
|
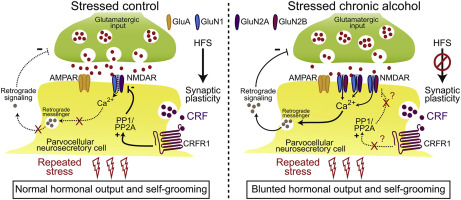
The chronic inability of alcoholics to effectively cope with relapse-inducing stressors has been linked to dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and corticotropin-releasing factor (CRF) signaling. However, the cellular mechanisms responsible for this dysregulation are yet to be identified. After exposure of male Sprague Dawley rats to chronic intermittent ethanol (CIE; 5-6 g/kg orally for 35 doses over 50 days) or water, followed by 40-60 days of protracted withdrawal, we investigated CIE effects on glutamatergic synaptic transmission, stress-induced plasticity, CRF- and ethanol-induced NMDAR inhibition using electrophysiological recordings in parvocellular neurosecretory cells (PNCs) of the paraventricular nucleus. We also assessed CIE effects on hypothalamic mRNA expression of CRF-related genes using real-time polymerase chain reaction, and on HPA axis function by measuring stress-induced increases in plasma adrenocorticotropic hormone, corticosterone, and self-grooming. In control rats, ethanol-mediated inhibition of NMDARs was prevented by CRF1 receptor (CRFR1) blockade with antalarmin, while CRF/CRFR1-mediated NMDAR blockade was prevented by intracellularly-applied inhibitor of phosphatases PP1/PP2A, okadaic acid, but not the selective striatal-enriched tyrosine protein phosphatase inhibitor, TC-2153. CIE exposure increased GluN2B subunit-dependent NMDAR function of PNCs. This was associated with the loss of both ethanol- and CRF-mediated NMDAR inhibition, and loss of stress-induced short-term potentiation of glutamatergic synaptic inputs, which could be reversed by intracellular blockade of NMDARs with MK801. CIE exposure also blunted the hormonal and self-grooming behavioral responses to repeated restraint stress. These findings suggest a cellular mechanism whereby chronic alcohol dysregulates the hormonal and behavioral responses to repetitive stressors by increasing NMDAR function and decreasing CRFR1 function.
中文翻译:

慢性酒精通过改变 CRF 和 NMDA 受体功能来破坏下丘脑对压力的反应。
酗酒者长期无法有效应对诱发复发的应激源,这与下丘脑-垂体-肾上腺 (HPA) 轴和促肾上腺皮质激素释放因子 (CRF) 信号失调有关。然而,导致这种失调的细胞机制尚未确定。在雄性 Sprague Dawley 大鼠暴露于慢性间歇乙醇(CIE;50 天内口服 5-6 克/千克,共 35 剂)或水,随后持续停药 40-60 天后,我们研究了 CIE 对谷氨酸能突触传递的影响,应激诱导的可塑性、CRF 和乙醇诱导的 NMDAR 抑制使用室旁核的细小细胞神经分泌细胞 (PNC) 中的电生理记录。我们还使用实时聚合酶链反应评估 CIE 对 CRF 相关基因的下丘脑 mRNA 表达的影响,并通过测量应激诱导的血浆促肾上腺皮质激素、皮质酮和自我修饰的增加来评估 CIE 对 HPA 轴功能的影响。在对照大鼠中,乙醇介导的 NMDAR 抑制被 antalarmin 的 CRF1 受体 (CRFR1) 阻断所阻止,而 CRF/CRFR1 介导的 NMDAR 阻断被细胞内应用的磷酸酶抑制剂 PP1/PP2A、冈田酸阻止,但不是选择性的富含纹状体的酪氨酸蛋白磷酸酶抑制剂,TC-2153。CIE 暴露增加了 PNC 的 GluN2B 亚基依赖性 NMDAR 功能。这与乙醇和 CRF 介导的 NMDAR 抑制作用的丧失以及应激诱导的谷氨酸能突触输入的短期增强作用的丧失有关,这可以通过用 MK801 对 NMDAR 进行细胞内阻断来逆转。CIE 暴露也削弱了对反复约束压力的荷尔蒙和自我梳理行为反应。这些发现表明了一种细胞机制,慢性酒精通过增加 NMDAR 功能和降低 CRFR1 功能来调节对重复性压力源的激素和行为反应。
更新日期:2020-02-12
中文翻译:

慢性酒精通过改变 CRF 和 NMDA 受体功能来破坏下丘脑对压力的反应。
酗酒者长期无法有效应对诱发复发的应激源,这与下丘脑-垂体-肾上腺 (HPA) 轴和促肾上腺皮质激素释放因子 (CRF) 信号失调有关。然而,导致这种失调的细胞机制尚未确定。在雄性 Sprague Dawley 大鼠暴露于慢性间歇乙醇(CIE;50 天内口服 5-6 克/千克,共 35 剂)或水,随后持续停药 40-60 天后,我们研究了 CIE 对谷氨酸能突触传递的影响,应激诱导的可塑性、CRF 和乙醇诱导的 NMDAR 抑制使用室旁核的细小细胞神经分泌细胞 (PNC) 中的电生理记录。我们还使用实时聚合酶链反应评估 CIE 对 CRF 相关基因的下丘脑 mRNA 表达的影响,并通过测量应激诱导的血浆促肾上腺皮质激素、皮质酮和自我修饰的增加来评估 CIE 对 HPA 轴功能的影响。在对照大鼠中,乙醇介导的 NMDAR 抑制被 antalarmin 的 CRF1 受体 (CRFR1) 阻断所阻止,而 CRF/CRFR1 介导的 NMDAR 阻断被细胞内应用的磷酸酶抑制剂 PP1/PP2A、冈田酸阻止,但不是选择性的富含纹状体的酪氨酸蛋白磷酸酶抑制剂,TC-2153。CIE 暴露增加了 PNC 的 GluN2B 亚基依赖性 NMDAR 功能。这与乙醇和 CRF 介导的 NMDAR 抑制作用的丧失以及应激诱导的谷氨酸能突触输入的短期增强作用的丧失有关,这可以通过用 MK801 对 NMDAR 进行细胞内阻断来逆转。CIE 暴露也削弱了对反复约束压力的荷尔蒙和自我梳理行为反应。这些发现表明了一种细胞机制,慢性酒精通过增加 NMDAR 功能和降低 CRFR1 功能来调节对重复性压力源的激素和行为反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号