当前位置:
X-MOL 学术
›
ACS Mater. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
P-Type Electrochemical Doping Can Occur by Cation Expulsion in a High-Performing Polymer for Organic Electrochemical Transistors
ACS Materials Letters ( IF 9.6 ) Pub Date : 2020-02-13 , DOI: 10.1021/acsmaterialslett.9b00501 Lucas Q. Flagg 1 , Connor G. Bischak 1 , Ramsess J. Quezada 1 , Jonathan W. Onorato 2 , Christine. K. Luscombe 1, 2, 3 , David S. Ginger 1, 3
ACS Materials Letters ( IF 9.6 ) Pub Date : 2020-02-13 , DOI: 10.1021/acsmaterialslett.9b00501 Lucas Q. Flagg 1 , Connor G. Bischak 1 , Ramsess J. Quezada 1 , Jonathan W. Onorato 2 , Christine. K. Luscombe 1, 2, 3 , David S. Ginger 1, 3
Affiliation
|
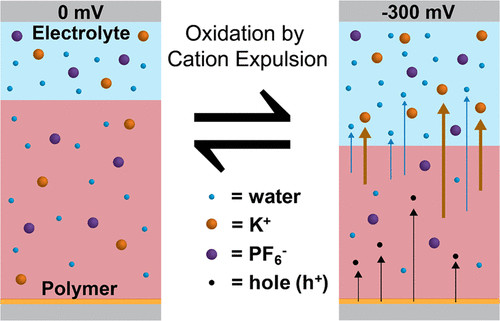
We investigate the mechanism of ion-dependent charge compensation during electrochemical oxidation (doping) of the model mixed ionic/electronic transporting polythiophene derivative poly(3-{[2-(2-methoxyethoxy)ethoxy]methyl}thiophene-2,5-diyl) (P3MEEMT). Using a combination of electrochemical quartz microbalance gravimetry and glow discharge optical emission spectroscopy, we show that charge compensation during polymer redox processes proceeds via a cation-dependent mechanism. For p-type polymer oxidation in certain electrolytes, charge compensation is achieved by both eventual injection of anions into the film, as well as initial expulsion of cations from the film. We compare doping mechanisms for a variety of electrolyte salts including potassium chloride, tetrabutylammonium chloride, potassium hexafluorophosphate (KPF6), and tetrabutylammonium hexafluorophosphate. For the electrolyte KPF6, both the cations and anions coexist in the water-swelled polymer even prior to application of electrical bias. Our data indicate that electrochemical doping (hole injection into the polymer and ionic charge compensation) proceeds via the following mechanism: (1) hydration of the neutral film by electrolyte (water, cations, anions), (2) cation (K+) expulsion from the film upon initial application of an oxidative bias, and (3) anion injection into the film at higher oxidation/doping levels (>∼2 × 1020/cm3). Understanding the mechanism of charge compensation during the doping process should allow for the design of improved mixed ionic/electronic conductors for use in applications ranging from organic supercapacitors and redox flow batteries to bioelectronic sensors, thermoelectrics, and devices for neuromorphic computing.
中文翻译:

在有机电化学晶体管的高性能聚合物中,阳离子驱除会发生P型电化学掺杂
我们研究模型混合离子/电子传输聚噻吩衍生物聚(3-{[2-(2-甲氧基乙氧基)乙氧基]甲基}噻吩-2,5-二基的电化学氧化(掺杂)过程中离子依赖性电荷补偿的机制)(P3MEEMT)。使用电化学石英微量天平重量分析法和辉光放电光发射光谱法的组合,我们表明聚合物氧化还原过程中的电荷补偿是通过阳离子依赖性机制进行的。对于某些电解质中的p型聚合物氧化,可以通过将阴离子最终注入薄膜中以及从薄膜中初始排出阳离子来实现电荷补偿。我们比较了各种电解质盐的掺杂机理,包括氯化钾,四丁基氯化铵,六氟磷酸钾(KPF 6)和四丁基六氟磷酸铵。对于电解质KPF 6,甚至在施加电偏压之前,阳离子和阴离子都共存于水溶胀的聚合物中。我们的数据表明,电化学掺杂(向聚合物中注入空穴和补偿离子电荷)是通过以下机制进行的:(1)电解质(水,阳离子,阴离子)使中性膜水化,(2 )排出阳离子(K +)从最初施加氧化偏压时的薄膜中去除,以及(3)以更高的氧化/掺杂水平(>〜2×10 20 / cm 3)。了解掺杂过程中的电荷补偿机制,应可以设计出改进的混合离子/电子导体,以用于从有机超级电容器和氧化还原液流电池到生物电子传感器,热电器件和神经形态计算设备的各种应用。
更新日期:2020-02-13
中文翻译:

在有机电化学晶体管的高性能聚合物中,阳离子驱除会发生P型电化学掺杂
我们研究模型混合离子/电子传输聚噻吩衍生物聚(3-{[2-(2-甲氧基乙氧基)乙氧基]甲基}噻吩-2,5-二基的电化学氧化(掺杂)过程中离子依赖性电荷补偿的机制)(P3MEEMT)。使用电化学石英微量天平重量分析法和辉光放电光发射光谱法的组合,我们表明聚合物氧化还原过程中的电荷补偿是通过阳离子依赖性机制进行的。对于某些电解质中的p型聚合物氧化,可以通过将阴离子最终注入薄膜中以及从薄膜中初始排出阳离子来实现电荷补偿。我们比较了各种电解质盐的掺杂机理,包括氯化钾,四丁基氯化铵,六氟磷酸钾(KPF 6)和四丁基六氟磷酸铵。对于电解质KPF 6,甚至在施加电偏压之前,阳离子和阴离子都共存于水溶胀的聚合物中。我们的数据表明,电化学掺杂(向聚合物中注入空穴和补偿离子电荷)是通过以下机制进行的:(1)电解质(水,阳离子,阴离子)使中性膜水化,(2 )排出阳离子(K +)从最初施加氧化偏压时的薄膜中去除,以及(3)以更高的氧化/掺杂水平(>〜2×10 20 / cm 3)。了解掺杂过程中的电荷补偿机制,应可以设计出改进的混合离子/电子导体,以用于从有机超级电容器和氧化还原液流电池到生物电子传感器,热电器件和神经形态计算设备的各种应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号