Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Vancomycin or Daptomycin With vs Without an Antistaphylococcal β-Lactam on Mortality, Bacteremia, Relapse, or Treatment Failure in Patients With MRSA Bacteremia
JAMA ( IF 63.1 ) Pub Date : 2020-02-11 , DOI: 10.1001/jama.2020.0103 Steven Y C Tong 1, 2 , David C Lye 3, 4, 5, 6 , Dafna Yahav 7, 8 , Archana Sud 9, 10 , J Owen Robinson 11, 12, 13, 14 , Jane Nelson 2 , Sophia Archuleta 15, 16 , Matthew A Roberts 17, 18 , Alan Cass 2 , David L Paterson 19 , Hong Foo 20 , Mical Paul 21, 22 , Stephen D Guy 23 , Adrian R Tramontana 23 , Genevieve B Walls 24 , Stephen McBride 24 , Narin Bak 25 , Niladri Ghosh 26 , Benjamin A Rogers 27, 28 , Anna P Ralph 2, 29 , Jane Davies 2, 29 , Patricia E Ferguson 30 , Ravindra Dotel 30, 31 , Genevieve L McKew 32, 33 , Timothy J Gray 32, 33 , Natasha E Holmes 34 , Simon Smith 35 , Morgyn S Warner 36, 37 , Shirin Kalimuddin 38, 39 , Barnaby E Young 3, 4 , Naomi Runnegar 40, 41 , David N Andresen 42, 43 , Nicholas A Anagnostou 44 , Sandra A Johnson 1 , Mark D Chatfield 2, 19 , Allen C Cheng 45, 46 , Vance G Fowler 47, 48 , Benjamin P Howden 34, 49 , Niamh Meagher 50 , David J Price 50, 51 , Sebastiaan J van Hal 52 , Matthew V N O'Sullivan 33, 53 , Joshua S Davis 2, 54 ,
JAMA ( IF 63.1 ) Pub Date : 2020-02-11 , DOI: 10.1001/jama.2020.0103 Steven Y C Tong 1, 2 , David C Lye 3, 4, 5, 6 , Dafna Yahav 7, 8 , Archana Sud 9, 10 , J Owen Robinson 11, 12, 13, 14 , Jane Nelson 2 , Sophia Archuleta 15, 16 , Matthew A Roberts 17, 18 , Alan Cass 2 , David L Paterson 19 , Hong Foo 20 , Mical Paul 21, 22 , Stephen D Guy 23 , Adrian R Tramontana 23 , Genevieve B Walls 24 , Stephen McBride 24 , Narin Bak 25 , Niladri Ghosh 26 , Benjamin A Rogers 27, 28 , Anna P Ralph 2, 29 , Jane Davies 2, 29 , Patricia E Ferguson 30 , Ravindra Dotel 30, 31 , Genevieve L McKew 32, 33 , Timothy J Gray 32, 33 , Natasha E Holmes 34 , Simon Smith 35 , Morgyn S Warner 36, 37 , Shirin Kalimuddin 38, 39 , Barnaby E Young 3, 4 , Naomi Runnegar 40, 41 , David N Andresen 42, 43 , Nicholas A Anagnostou 44 , Sandra A Johnson 1 , Mark D Chatfield 2, 19 , Allen C Cheng 45, 46 , Vance G Fowler 47, 48 , Benjamin P Howden 34, 49 , Niamh Meagher 50 , David J Price 50, 51 , Sebastiaan J van Hal 52 , Matthew V N O'Sullivan 33, 53 , Joshua S Davis 2, 54 ,
Affiliation
|
Importance
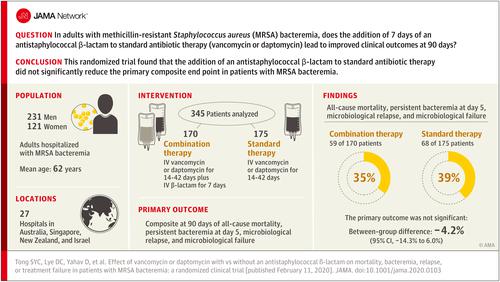
Methicillin-resistant Staphylococcus aureus (MRSA) bacteremia is associated with mortality of more than 20%. Combining standard therapy with a β-lactam antibiotic has been associated with reduced mortality, although adequately powered randomized clinical trials of this intervention have not been conducted. Objective
To determine whether combining an antistaphylococcal β-lactam with standard therapy is more effective than standard therapy alone in patients with MRSA bacteremia. Design, Setting, and Participants
Open-label, randomized clinical trial conducted at 27 hospital sites in 4 countries from August 2015 to July 2018 among 352 hospitalized adults with MRSA bacteremia. Follow-up was complete on October 23, 2018. Interventions
Participants were randomized to standard therapy (intravenous vancomycin or daptomycin) plus an antistaphylococcal β-lactam (intravenous flucloxacillin, cloxacillin, or cefazolin) (n = 174) or standard therapy alone (n = 178). Total duration of therapy was determined by treating clinicians and the β-lactam was administered for 7 days. Main Outcomes and Measures
The primary end point was a 90-day composite of mortality, persistent bacteremia at day 5, microbiological relapse, and microbiological treatment failure. Secondary outcomes included mortality at days 14, 42, and 90; persistent bacteremia at days 2 and 5; acute kidney injury (AKI); microbiological relapse; microbiological treatment failure; and duration of intravenous antibiotics. Results
The data and safety monitoring board recommended early termination of the study prior to enrollment of 440 patients because of safety. Among 352 patients randomized (mean age, 62.2 [SD, 17.7] years; 121 women [34.4%]), 345 (98%) completed the trial. The primary end point was met by 59 (35%) with combination therapy and 68 (39%) with standard therapy (absolute difference, -4.2%; 95% CI, -14.3% to 6.0%). Seven of 9 prespecified secondary end points showed no significant difference. For the combination therapy vs standard therapy groups, all-cause 90-day mortality occurred in 35 (21%) vs 28 (16%) (difference, 4.5%; 95% CI, -3.7% to 12.7%); persistent bacteremia at day 5 was observed in 19 of 166 (11%) vs 35 of 172 (20%) (difference, -8.9%; 95% CI, -16.6% to -1.2%); and, excluding patients receiving dialysis at baseline, AKI occurred in 34 of 145 (23%) vs 9 of 145 (6%) (difference, 17.2%; 95% CI, 9.3%-25.2%). Conclusions and Relevance
Among patients with MRSA bacteremia, addition of an antistaphylococcal β-lactam to standard antibiotic therapy with vancomycin or daptomycin did not result in significant improvement in the primary composite end point of mortality, persistent bacteremia, relapse, or treatment failure. Early trial termination for safety concerns and the possibility that the study was underpowered to detect clinically important differences in favor of the intervention should be considered when interpreting the findings. Trial Registration
ClinicalTrials.gov Identifier: NCT02365493.
中文翻译:

万古霉素或达托霉素联合与不联合抗葡萄球菌 β-内酰胺对 MRSA 菌血症患者死亡率、菌血症、复发或治疗失败的影响
重要性 耐甲氧西林金黄色葡萄球菌 (MRSA) 菌血症与超过 20% 的死亡率相关。将标准疗法与 β-内酰胺类抗生素相结合可降低死亡率,尽管尚未进行这种干预措施的充分把握的随机临床试验。目的 确定在 MRSA 菌血症患者中,抗葡萄球菌 β-内酰胺类药物联合标准疗法是否比单独标准疗法更有效。设计、设置和参与者 2015 年 8 月至 2018 年 7 月,在 4 个国家的 27 个医院地点对 352 名患有 MRSA 菌血症的住院成人进行了开放标签、随机临床试验。随访于2018年10月23日完成。干预 参与者随机接受标准治疗(静脉内万古霉素或达托霉素)加抗葡萄球菌 β-内酰胺(静脉内氟氯西林、氯唑西林或头孢唑林)(n = 174)或单独标准治疗(n = 178)。治疗的总持续时间由治疗临床医生确定,β-内酰胺给药 7 天。主要结果和测量主要终点是死亡率、第 5 天持续菌血症、微生物学复发和微生物学治疗失败的 90 天复合终点。次要结果包括第 14、42 和 90 天的死亡率;第 2 天和第 5 天持续菌血症;急性肾损伤 (AKI); 微生物复发;微生物处理失败;和静脉注射抗生素的持续时间。结果 出于安全考虑,数据和安全监察委员会建议在招募 440 名患者之前提前终止研究。在随机分配的 352 名患者中(平均年龄 62.2 [SD,17.7] 岁;121 名女性 [34.4%]),345 名 (98%) 完成了试验。59 名(35%)联合治疗达到主要终点,标准治疗 68 名(39%)达到主要终点(绝对差异,-4.2%;95% CI,-14.3% 至 6.0%)。9 个预设的次要终点中有 7 个没有显着差异。对于联合治疗组与标准治疗组,90 天全因死亡率分别为 35 (21%) 和 28 (16%)(差异,4.5%;95% CI,-3.7% 至 12.7%);166 人中有 19 人(11%)与 172 人中有 35 人(20%)在第 5 天观察到持续性菌血症(差异,-8.9%;95% CI,-16.6% 至 -1.2%);并且,不包括在基线时接受透析的患者,145 人中的 34 人 (23%) 与 145 人中的 9 人 (6%) 发生 AKI(差异,17.2%;95% CI,9.3%-25.2%)。结论和相关性 在 MRSA 菌血症患者中,在万古霉素或达托霉素标准抗生素治疗中加入抗葡萄球菌 β-内酰胺类药物并未显着改善死亡率、持续性菌血症、复发或治疗失败等主要复合终点。在解释研究结果时,应考虑出于安全考虑而提前终止试验以及该研究不足以检测有利于干预的临床重要差异的可能性。试验注册 ClinicalTrials.gov 标识符:NCT02365493。在使用万古霉素或达托霉素的标准抗生素治疗中添加抗葡萄球菌 β-内酰胺类药物,并未显着改善死亡率、持续性菌血症、复发或治疗失败等主要复合终点。在解释研究结果时,应考虑出于安全考虑而提前终止试验以及该研究不足以检测有利于干预的临床重要差异的可能性。试验注册 ClinicalTrials.gov 标识符:NCT02365493。在使用万古霉素或达托霉素的标准抗生素治疗中添加抗葡萄球菌 β-内酰胺类药物,并未显着改善死亡率、持续性菌血症、复发或治疗失败等主要复合终点。在解释研究结果时,应考虑出于安全考虑而提前终止试验以及该研究不足以检测有利于干预的临床重要差异的可能性。试验注册 ClinicalTrials.gov 标识符:NCT02365493。在解释研究结果时,应考虑出于安全考虑而提前终止试验以及该研究不足以检测有利于干预的临床重要差异的可能性。试验注册 ClinicalTrials.gov 标识符:NCT02365493。在解释研究结果时,应考虑出于安全考虑而提前终止试验以及该研究不足以检测有利于干预的临床重要差异的可能性。试验注册 ClinicalTrials.gov 标识符:NCT02365493。
更新日期:2020-02-11
中文翻译:

万古霉素或达托霉素联合与不联合抗葡萄球菌 β-内酰胺对 MRSA 菌血症患者死亡率、菌血症、复发或治疗失败的影响
重要性 耐甲氧西林金黄色葡萄球菌 (MRSA) 菌血症与超过 20% 的死亡率相关。将标准疗法与 β-内酰胺类抗生素相结合可降低死亡率,尽管尚未进行这种干预措施的充分把握的随机临床试验。目的 确定在 MRSA 菌血症患者中,抗葡萄球菌 β-内酰胺类药物联合标准疗法是否比单独标准疗法更有效。设计、设置和参与者 2015 年 8 月至 2018 年 7 月,在 4 个国家的 27 个医院地点对 352 名患有 MRSA 菌血症的住院成人进行了开放标签、随机临床试验。随访于2018年10月23日完成。干预 参与者随机接受标准治疗(静脉内万古霉素或达托霉素)加抗葡萄球菌 β-内酰胺(静脉内氟氯西林、氯唑西林或头孢唑林)(n = 174)或单独标准治疗(n = 178)。治疗的总持续时间由治疗临床医生确定,β-内酰胺给药 7 天。主要结果和测量主要终点是死亡率、第 5 天持续菌血症、微生物学复发和微生物学治疗失败的 90 天复合终点。次要结果包括第 14、42 和 90 天的死亡率;第 2 天和第 5 天持续菌血症;急性肾损伤 (AKI); 微生物复发;微生物处理失败;和静脉注射抗生素的持续时间。结果 出于安全考虑,数据和安全监察委员会建议在招募 440 名患者之前提前终止研究。在随机分配的 352 名患者中(平均年龄 62.2 [SD,17.7] 岁;121 名女性 [34.4%]),345 名 (98%) 完成了试验。59 名(35%)联合治疗达到主要终点,标准治疗 68 名(39%)达到主要终点(绝对差异,-4.2%;95% CI,-14.3% 至 6.0%)。9 个预设的次要终点中有 7 个没有显着差异。对于联合治疗组与标准治疗组,90 天全因死亡率分别为 35 (21%) 和 28 (16%)(差异,4.5%;95% CI,-3.7% 至 12.7%);166 人中有 19 人(11%)与 172 人中有 35 人(20%)在第 5 天观察到持续性菌血症(差异,-8.9%;95% CI,-16.6% 至 -1.2%);并且,不包括在基线时接受透析的患者,145 人中的 34 人 (23%) 与 145 人中的 9 人 (6%) 发生 AKI(差异,17.2%;95% CI,9.3%-25.2%)。结论和相关性 在 MRSA 菌血症患者中,在万古霉素或达托霉素标准抗生素治疗中加入抗葡萄球菌 β-内酰胺类药物并未显着改善死亡率、持续性菌血症、复发或治疗失败等主要复合终点。在解释研究结果时,应考虑出于安全考虑而提前终止试验以及该研究不足以检测有利于干预的临床重要差异的可能性。试验注册 ClinicalTrials.gov 标识符:NCT02365493。在使用万古霉素或达托霉素的标准抗生素治疗中添加抗葡萄球菌 β-内酰胺类药物,并未显着改善死亡率、持续性菌血症、复发或治疗失败等主要复合终点。在解释研究结果时,应考虑出于安全考虑而提前终止试验以及该研究不足以检测有利于干预的临床重要差异的可能性。试验注册 ClinicalTrials.gov 标识符:NCT02365493。在使用万古霉素或达托霉素的标准抗生素治疗中添加抗葡萄球菌 β-内酰胺类药物,并未显着改善死亡率、持续性菌血症、复发或治疗失败等主要复合终点。在解释研究结果时,应考虑出于安全考虑而提前终止试验以及该研究不足以检测有利于干预的临床重要差异的可能性。试验注册 ClinicalTrials.gov 标识符:NCT02365493。在解释研究结果时,应考虑出于安全考虑而提前终止试验以及该研究不足以检测有利于干预的临床重要差异的可能性。试验注册 ClinicalTrials.gov 标识符:NCT02365493。在解释研究结果时,应考虑出于安全考虑而提前终止试验以及该研究不足以检测有利于干预的临床重要差异的可能性。试验注册 ClinicalTrials.gov 标识符:NCT02365493。











































 京公网安备 11010802027423号
京公网安备 11010802027423号