当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Zn-Catalyzed Nicotinate-Directed Transamidations in Peptide Synthesis
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-20 , DOI: 10.1021/acscatal.9b05074 Karlijn Hollanders 1, 2 , Evelien Renders 2 , Charlène Gadais 1, 2 , Dario Masullo 2 , Laurent Van Raemdonck 2 , Clarence C. D. Wybon 2 , Charlotte Martin 1 , Wouter A. Herrebout 3 , Bert U. W. Maes 2 , Steven Ballet 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-20 , DOI: 10.1021/acscatal.9b05074 Karlijn Hollanders 1, 2 , Evelien Renders 2 , Charlène Gadais 1, 2 , Dario Masullo 2 , Laurent Van Raemdonck 2 , Clarence C. D. Wybon 2 , Charlotte Martin 1 , Wouter A. Herrebout 3 , Bert U. W. Maes 2 , Steven Ballet 1
Affiliation
|
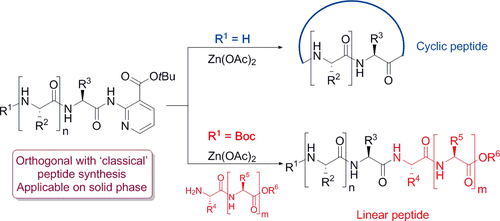
A chemoselective and catalytic transamidation for peptide synthesis is described. Transamidation under Zn catalysis is chemoselectively achieved by amino acid amide/peptidic amide derivatization with a tert-butyl nicotinate (tBu-nic) directing group. The directing group could be easily introduced on protected amino acid amides via Pd-catalyzed amidation with tert-butyl 2-chloronicotinate (tBu-nicCl). Under standard peptide coupling/deprotection conditions, the tBu-nic-equipped amino acid amides proved to be fully inert, allowing them to be easily built-in in complex molecules. The disclosed method was evaluated in the synthesis of diverse dipeptides, in dipeptide segment coupling, in side-chain modification of a solid-supported tetra-/pentapeptide, and in the macrocyclization of a heptapeptide.
中文翻译:

锌催化的烟酸酯定向转氨作用的肽合成
描述了用于肽合成的化学选择和催化转氨基。锌催化下的氨基转移是通过烟酰胺叔丁酸酯(t Bu-nic)导向基团的氨基酸酰胺/肽酰胺衍生化而实现的。通过用2-氯烟酸叔丁酯(t Bu-nicCl)进行Pd催化的酰胺化,可以容易地将导向基团引入到受保护的氨基酸酰胺上。在标准肽偶联/去保护条件下,t带有Bunic的氨基酸酰胺被证明是完全惰性的,使它们易于内置在复杂分子中。在各种二肽的合成,二肽链段偶联,固相支持的四肽/五肽的侧链修饰以及七肽的大环化中评估了所公开的方法。
更新日期:2020-03-21
中文翻译:

锌催化的烟酸酯定向转氨作用的肽合成
描述了用于肽合成的化学选择和催化转氨基。锌催化下的氨基转移是通过烟酰胺叔丁酸酯(t Bu-nic)导向基团的氨基酸酰胺/肽酰胺衍生化而实现的。通过用2-氯烟酸叔丁酯(t Bu-nicCl)进行Pd催化的酰胺化,可以容易地将导向基团引入到受保护的氨基酸酰胺上。在标准肽偶联/去保护条件下,t带有Bunic的氨基酸酰胺被证明是完全惰性的,使它们易于内置在复杂分子中。在各种二肽的合成,二肽链段偶联,固相支持的四肽/五肽的侧链修饰以及七肽的大环化中评估了所公开的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号