当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkylation of γ-Azaproline Creates Conformationally Adaptable Proline Derivatives for pH-Responsive Collagen Triple Helices.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-03-26 , DOI: 10.1002/chem.201905768 Matthew R Aronoff 1 , Jasmine Egli 1 , Adeline Schmitt 1 , Helma Wennemers 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-03-26 , DOI: 10.1002/chem.201905768 Matthew R Aronoff 1 , Jasmine Egli 1 , Adeline Schmitt 1 , Helma Wennemers 1
Affiliation
|
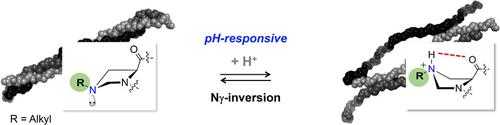
Cγ-substituted proline derivatives are valuable tools for developing functionalized collagen peptides for biological and materials investigations, yet the stereochemistry at Cγ can produce undesired steric or stereoelectronic constraints. Alkylated γ-azaproline (γ-azPro) derivatives are proline mimetics that lack a stereogenic center at the γ-position of the ring and can thus utilize the invertibility of nitrogen to adapt their conformation. NMR spectroscopic analyses and DFT calculations highlight how alkylated g-azPro derivatives are conformationally dynamic and adopt conformational preferences through ring pucker flip along with nitrogen inversion. Lastly, incorporation of alkylated g-azPro into collagen peptides produced functionalized pH-responsive triple helices with similar thermal stabilities, regardless of their placement in the Xaa or Yaa position within the characteristic Xaa-Yaa-Gly repeating unitof collagen peptides.
中文翻译:

γ-氮杂脯氨酸的烷基化可形成构型适应性的脯氨酸衍生物,用于pH敏感型胶原三重螺旋。
Cγ取代的脯氨酸衍生物是开发用于生物学和材料研究的功能化胶原蛋白肽的有价值的工具,但Cγ处的立体化学会产生不良的空间或立体电子约束。烷基化的γ-氮杂脯氨酸(γ-azPro)衍生物是脯氨酸模拟物,在环的γ位缺少立体异构中心,因此可以利用氮的可逆性来适应其构象。NMR光谱分析和DFT计算突出了烷基化的g-azPro衍生物的构象动力学,并通过环褶皱翻转和氮转化采用构象偏好。最后,将烷基化的g-azPro掺入胶原蛋白肽中可产生具有类似热稳定性的功能化pH响应三重螺旋,
更新日期:2020-03-27
中文翻译:

γ-氮杂脯氨酸的烷基化可形成构型适应性的脯氨酸衍生物,用于pH敏感型胶原三重螺旋。
Cγ取代的脯氨酸衍生物是开发用于生物学和材料研究的功能化胶原蛋白肽的有价值的工具,但Cγ处的立体化学会产生不良的空间或立体电子约束。烷基化的γ-氮杂脯氨酸(γ-azPro)衍生物是脯氨酸模拟物,在环的γ位缺少立体异构中心,因此可以利用氮的可逆性来适应其构象。NMR光谱分析和DFT计算突出了烷基化的g-azPro衍生物的构象动力学,并通过环褶皱翻转和氮转化采用构象偏好。最后,将烷基化的g-azPro掺入胶原蛋白肽中可产生具有类似热稳定性的功能化pH响应三重螺旋,









































 京公网安备 11010802027423号
京公网安备 11010802027423号