当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The interaction of CO with a copper(II) chloride oxy-chlorination catalyst
Faraday Discussions ( IF 3.3 ) Pub Date : 2020-2-11 , DOI: 10.1039/d0fd00014k Shaoliang Guan 1, 2, 3, 4, 5 , Giovanni E. Rossi 2, 5, 6, 7 , John M. Winfield 2, 5, 6, 7 , Claire Wilson 2, 5, 6, 7 , Donald MacLaren 5, 6, 7, 8 , David J. Morgan 1, 2, 3, 4, 5 , Philip R. Davies 1, 2, 3, 4, 5 , David J. Willock 1, 2, 3, 4, 5 , David Lennon 2, 5, 6, 7
Faraday Discussions ( IF 3.3 ) Pub Date : 2020-2-11 , DOI: 10.1039/d0fd00014k Shaoliang Guan 1, 2, 3, 4, 5 , Giovanni E. Rossi 2, 5, 6, 7 , John M. Winfield 2, 5, 6, 7 , Claire Wilson 2, 5, 6, 7 , Donald MacLaren 5, 6, 7, 8 , David J. Morgan 1, 2, 3, 4, 5 , Philip R. Davies 1, 2, 3, 4, 5 , David J. Willock 1, 2, 3, 4, 5 , David Lennon 2, 5, 6, 7
Affiliation
|
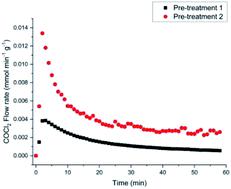
The interaction of CO with an attapulgite-supported, KCl modified CuCl2 catalyst has previously been examined using a combination of XANES, EXAFS and DFT calculations. Exposing the catalyst to CO at elevated temperatures leads to the formation of CO2 as the only identifiable product. However, phosgene production can be induced by a catalyst pre-treatment stage, where the supported CuCl2 sample is exposed to a diluted stream of dichlorine; subsequent CO exposure at ∼643 K then leads to phosgene production. This communication describes a series of FTIR based micro-reactor measurements, coupled with characterisation measurements utilising TEM, XRD and XPS to define the nature of the catalyst at different stages of the reaction coordinate. The CuCl2 catalyst is able to support Deacon activity 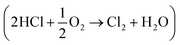 , establishing this work with the possibility of utilising the oxy-chlorination of CO to produce phosgene. Continuous dosing of CO at elevated temperatures over the chlorine pre-dosed CuCl2 catalyst shows diminishing phosgene production as a function of time-on-stream, indicating surface chlorine supply to be rate-limiting under the reaction conditions studied. A pictorial reaction scheme is proposed to account for the surface chemistry observed.
, establishing this work with the possibility of utilising the oxy-chlorination of CO to produce phosgene. Continuous dosing of CO at elevated temperatures over the chlorine pre-dosed CuCl2 catalyst shows diminishing phosgene production as a function of time-on-stream, indicating surface chlorine supply to be rate-limiting under the reaction conditions studied. A pictorial reaction scheme is proposed to account for the surface chemistry observed.
中文翻译:

CO与氯化铜(II)氧氯化催化剂的相互作用
以前已经使用XANES,EXAFS和DFT计算的组合检查了CO与凹凸棒石负载的KCl改性CuCl 2催化剂之间的相互作用。在升高的温度下将催化剂暴露于CO导致形成CO 2作为唯一可识别的产物。但是,光气的产生可以通过催化剂预处理阶段进行,在该阶段中,将负载的CuCl 2样品暴露于稀释的二氯流中;随后在约643 K下暴露于一氧化碳,导致产生光气。该通信描述了一系列基于FTIR的微反应器测量,以及利用TEM,XRD和XPS进行表征测量以定义催化剂在反应坐标不同阶段的性质的测量。氯化铜2催化剂能够支持迪肯活性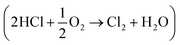 ,从而可以利用CO的氧氯化生成光气来开展这项工作。在预先添加氯的CuCl 2催化剂上,在高温下连续定量添加CO显示出光气产量随运行时间而变,表明在所研究的反应条件下,表面氯的供应受到速率的限制。提出了图示反应方案以说明所观察到的表面化学。
,从而可以利用CO的氧氯化生成光气来开展这项工作。在预先添加氯的CuCl 2催化剂上,在高温下连续定量添加CO显示出光气产量随运行时间而变,表明在所研究的反应条件下,表面氯的供应受到速率的限制。提出了图示反应方案以说明所观察到的表面化学。
更新日期:2020-02-11
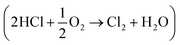 , establishing this work with the possibility of utilising the oxy-chlorination of CO to produce phosgene. Continuous dosing of CO at elevated temperatures over the chlorine pre-dosed CuCl2 catalyst shows diminishing phosgene production as a function of time-on-stream, indicating surface chlorine supply to be rate-limiting under the reaction conditions studied. A pictorial reaction scheme is proposed to account for the surface chemistry observed.
, establishing this work with the possibility of utilising the oxy-chlorination of CO to produce phosgene. Continuous dosing of CO at elevated temperatures over the chlorine pre-dosed CuCl2 catalyst shows diminishing phosgene production as a function of time-on-stream, indicating surface chlorine supply to be rate-limiting under the reaction conditions studied. A pictorial reaction scheme is proposed to account for the surface chemistry observed.
中文翻译:

CO与氯化铜(II)氧氯化催化剂的相互作用
以前已经使用XANES,EXAFS和DFT计算的组合检查了CO与凹凸棒石负载的KCl改性CuCl 2催化剂之间的相互作用。在升高的温度下将催化剂暴露于CO导致形成CO 2作为唯一可识别的产物。但是,光气的产生可以通过催化剂预处理阶段进行,在该阶段中,将负载的CuCl 2样品暴露于稀释的二氯流中;随后在约643 K下暴露于一氧化碳,导致产生光气。该通信描述了一系列基于FTIR的微反应器测量,以及利用TEM,XRD和XPS进行表征测量以定义催化剂在反应坐标不同阶段的性质的测量。氯化铜2催化剂能够支持迪肯活性
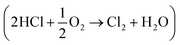 ,从而可以利用CO的氧氯化生成光气来开展这项工作。在预先添加氯的CuCl 2催化剂上,在高温下连续定量添加CO显示出光气产量随运行时间而变,表明在所研究的反应条件下,表面氯的供应受到速率的限制。提出了图示反应方案以说明所观察到的表面化学。
,从而可以利用CO的氧氯化生成光气来开展这项工作。在预先添加氯的CuCl 2催化剂上,在高温下连续定量添加CO显示出光气产量随运行时间而变,表明在所研究的反应条件下,表面氯的供应受到速率的限制。提出了图示反应方案以说明所观察到的表面化学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号